This Citizen's Petition for the labeling of beer and wine has
been submitted to the BATF
January 27, 1996
Bureau of Alcohol, Tobacco, and Firearms
Office of Regulation Enforcement Programs
650 Massachusetts Ave. NW
Washington, DC 20266
CITIZEN PETITION
The undersigned submits this Citizen Petition under the
relevant statutory section of the Public Health Service Act, or
under any other statutory provision for which authority has been
delegated to the Bureau of Alcohol, Tobacco, and Firearms, to
request the BATF to lobby Congress and the President, or take
other actions, to require the labeling of beer and wine
concerning their magnesium content.
ACTION REQUESTED
I request that the Bureau of Alcohol, Tobacco, and Firearms
lobby Congress and the President, or take other actions, to
require the labeling of beer and wine concerning their magnesium
content, for the purpose of saving about 26,000 lives per annum.
The following is a sample of the kind of label that we request
for a beer or wine containing 110 mg/L Mg, with a nutrition
warning that alcohol causes rapid loss of magnesium:
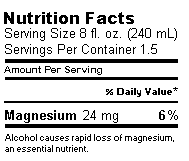
An alternative wording and layout for a magnesium nutrition
label would be:

1. Magnesium deficiency appears to be causing 215,000 U. S.
deaths per annum, as outlined in related petitions to the FDA
including 94P-0361/CP1, 95P-0381/CP1, 94P-0361/CP2. The
magnesium-deficiency problem is a highly technical nutrition
problem, so BATF should coordinate with FDA and draw on the FDA's
nutritional expertise. These petitions and significant other
information about the magnesium deficiency problem are available
to BATF at http://www.mgwater.com/
2. The current lack of nutrition labeling for beer and wine
constitutes a health hazard because some beers contain up to 250
mg/L magnesium (1), and some wines contain up to 245 mg/L
magnesium (2,18). Consumers who take magnesium supplements may
already be getting 1,000 mg/day of magnesium, so if such a
consumer drank an un-labelled liter of beer or wine containing
245-250 mg magnesium, they would very likely experience an
unwanted laxative effect. Dave Radzanowski, Vice President of the
Siebel Institute of Brewing Technology, was quoted in Beverage
Industry magazine, May 1995, p 24, "Most (American) brewers try
to limit magnesium in their water sources because, combined with
sulphates in beer, it tends to have a laxative effect". As
consumers become aware of the magnesium deficiency problem, they
are likely to take Mg supplements in greater numbers and
quantities, so it will be increasingly necessary for consumers to
know the Mg content of beer and wine to prevent untimely
defecations which would be a health hazard.
3. I had a laboratory test done of the leading American brand
of beer (Budweiser), as representative of American mega-brews,
and found that it contains only 34 mg/L magnesium (3), compared
with up to 250 mg/L magnesium in some European brews. British
beers have Mg levels ranging from 60-200 mg/L, German beers from
75-250 mg/L, and Lagers 34-162 mg/L (1). Apparently the American
mega-brews are relatively Mg-poor, just as American bottled
waters are relatively Mg-poor, compared to European brews and
bottled waters. Nutrition labeling is a necessary part of the
solution to these deficiencies.
4. It has been well documented that Americans are Mg deficient
(4,5,6,7,8,9,10,11,12, 13,14,15,16), and most Americans do not
take supplements, particularly the uneducated classes who are the
biggest beer drinkers. Many men consider it wimpy to take a
supplement, but wouldn't mind drinking a naturally Mg-rich beer.
Alcohol causes rapid excretion of magnesium (17), but
nevertheless, drinking an Mg-rich beer containing 250 mg/L
magnesium is generally better than drinking an Mg-poor beer with
only 34 mg/L magnesium.
5. I have heard a complaint from a micro-brewer that he was
prohibited by the FDA and BATF (Bureau of Alcohol, Tobacco, and
Firearms) from nutrition-labeling his brew with all the natural
vitamins and minerals it contained. It appears that Mg-poor
mega-brewers have prevented competition from Mg-rich
micro-brewers by successfully lobbying BATF to exclude beer and
wine from the nutrition labeling laws. The excuse has been that
Americans should not look to alcoholic beverages as a source of
nutrition; this argument is hollow, in light of the numerous
studies suggesting a cardio-benefit from moderate consumption of
beer or wine. It is likely that the cardio-benefit may result in
part from the magnesium and other nutrients contained in some
beers or wine. The FDA/BATF are preventing even the most careful
consumers from making informed decisions.
6. Another View is offered by Brewmaster A.J. Delange, who
writes, "I and lots of other beer-lovers hope that you succeed in
getting the FDA to put nutrition labels on beer. The FDA/BATF are
preventing the consumer from knowing what the consumers of any
other product are entitled to know, and that is unconscionable."
A. J. Delange's research on how to synthesize selected water
profiles for making Mg-rich drinking water and beer is given at
the Magnesium Web Site.
7. Beverage Industry magazine's Annual Manual 1995-1996 shows
in tables on page 18 that annual American consumption of beer per
capita is 22.5 gallons, for a Share of Stomach of 12.3%. In my
paper, Calculations of American Deaths Caused by Magnesium
Deficiency, As Projected From International Data (19), the point
was made that apparently 215,000 American deaths are caused each
year by magnesium deficiency. Mg-rich beer could apparently save
about 12.3% of those deaths, or about 26,445 lives per annum,
which is a lot more than the deaths at Waco and Ruby Ridge.
Nutrition labeling of beer and wine is a necessary step toward
resolving the Mg-deficiency catastrophe.
ENVIRONMENTAL IMPACT
I claim a categorical exclusion under paragraph 25.24 of 21
CFR.
UNFAVORABLE INFORMATION
1. None
CERTIFICATION
The Undersigned certifies, that, to the best knowledge and
belief of the Undersigned, this petition includes all information
and views on which the petition relies, and that it includes
representative data and information known to the petitioner which
are unfavorable to the petition.
(Signature)_________________________________________________
PAUL W. MASON
P O Box 1199
Livermore, CA 94551
tel: (408) 897-3023
fax: (408) 897-3028
REFERENCES AND/OR ENCLOSURES
1. "Malting and Brewing Science", by Briggs, Hough, Stevens,
and Young. Vol II, p 779
2. "Metal Content of California Wines", C. S. Ough, E.A.
Cromwell, J. Benz. Journal of Food Science, Vol 47, p 827.
(1982)
3. Alpha Analytical Laboratory, Batch 95-0918-018 9/18/95
4. Absorption and Excretion of Magnesium; Gastrointestinal
Absorption of Magnesium; Renal Excretion of Magnesium; etc.:
1984. (Text supplied with Docket 94P-0361/CP1)
5. Anderson, T. W.; Neri, L. C.; Schreiber, G. B.; Talbot, F.
D. F.; Zdrojewski, A.: Ischemic heart disease, water hardness and
myocardial magnesium: CMA Journal: Aug. 9, 1975. (Text supplied
with Docket 94P-0361/CP1)
6. Durlach, J.: Recommended dietary amounts of magnesium: Mg
RDA: Magnesium Research: 1989. (Text supplied with Docket
94P-0361/CP1)
7. Karppanen, H.; Tanskanen, A.; Tuomilehto, J.; Puska, P.;
Vuori, J.; Jantti, V.; Seppanen, M.: Safety and effects of
potassium- and magnesium-containing low sodium salt mixtures:
Journal of Cardiovascular Pharmacology: 1984. (Text supplied with
Docket 94P-0361/CP1)
8. Karppanen, Heikki: Epidemiological studies on the
relationship between magnesium intake and cardiovascular
diseases; Artery: 1981. (Text supplied with Docket
94P-0361/CP1)
9. Marier, J. R.: Magnesium Content of the Food Supply in the
Modern-Day World: 1986. (Text supplied with Docket
94P-0361/CP1)
10. Molloy, D. W.; Dhingra, S.; Solven, F.; Wilson, A.;
Mccarthy, D. S.: Hypomagnesemia and respiratory muscle power:
Concise Clinical Studies: 1984. (Text supplied with Docket
94P-0361/CP1)
11. Rayssiguier, Y.; Gueux, E.: Magnesium and lipids in
cardiovascular disease: Journal of the American College of
Nutrition: 1986. (Text supplied with Docket 94P-0361/CP1)
12. Reinhard, R. A.; Desbiens, N. A.: Hypomagnesemia in
patients entering the ICU: Critical Care Medicine: 1985. (Text
supplied with Docket 94P-0361/CP1)
13. Ryzen, E.; Wagers, P. W.; Singer, F. R.; Rude, R. K.;
Magnesium deficiency in a medical ICU population: Critical Care
Medicine: 1985. (Text supplied with Docket 94P-0361/CP1)
14. Seelig, M. S.: The requirement of magnesium by the normal
adult; summary and analysis of published data: American Journal
of Clinical Nutrition: Vol. 14: June 1964. (Text supplied with
Docket 94P-0361/CP1)
15. Tzivoni, D.; Keren, A.: Suppression of ventricular
arrhythmias by magnesium: The American Journal of Cardiology:
June 1, 1990. (Text supplied with Docket 94P-0361/CP1)
16. Wong, E. T.; Rude, R. K.; Singer, F. R.; Shaw, S. T.: A
high prevalence of hypomagnesemia and hypermagnesemia in
hospitalized patients: American Society of Clinical Pathologists:
March 1983. (Text supplied with Docket 94P-0361/CP1)
17. Kalbfleish, J. M., et al. Effects of Ethanol
Administration on Urinary Excretion of magnesium and other
electrolytes in alcoholic and normal subjects. Journal of
Clinical Investigations. Vol. 42. 1963.
18. Interesse F. S., D'Avella G., Alloggio V., Lamparelli F.
Mineral Contents of Some Southern Italian Wines. Z. Lebenam
Upscro Forsch (1985) 181:470-474
19. Mason, P.; Mason, J.: Calculations of American Deaths
Caused by Magnesium Deficiency, As Projected From International
Data: Jan. 1994.
NOTE: A similar petition was originally submitted to the FDA
on Oct. 22, 1995; on 1/26/96 Jerry McCowin at the FDA's Office of
Food Labeling informed me that FDA and BATF had joint
responsibility for the nutrition labeling of beer and wine, and
that a recent meeting had determined that BATF would be the lead
agency concerned with nutrition labeling of beer and wine. Mr.
McCowin further gave me the option of withdrawing Citizen
Petition 95P-0353/CP 1 from the FDA and re-submitting it to the
BATF, or else he would deny the petition on the grounds that FDA
was the incorrect agency for consideration of it. Given that
ultimatum, I chose to withdraw FDA 95P-0353/CP 1 and resubmit to
BATF.
This page was first uploaded on to The Magnesium Web Site on
February 14, 1996
http://www.mgwater.com/

