Magnesium Research (1993) 6, 1, 11-19
ORIGINAL ARTICLE
Audiogenic seizures in magnesium-deficient mice: effects of
magnesium pyrrolidone-2-carboxylate, magnesium acetyltaurinate,
magnesium chloride and vitamin B-6
Pierre Bac1, Christine Herrenknecht, Pierre
Binet1 and Jean Durlach2
1Faculté de Pharmacie, rue J.B.
C1ément; 2S.D.R.M. Hôpital Saint-Vincent
de Paul, Avenue Denfert-Rochereau, Paris, France
Summary: Magnesium deficiency in
mice causes and increases audiogenic seizures. This effect was
reversed by oral administration of magnesium acetyltaurinate
(ATaMg), magnesium pyrrolidone-2-carboxylate (PCMH),
MgCl2. When treatment was discontinued, audiogenic
seizures recurred only in the groups treated with PCMH or
MgCl2. Following intraperitoneal administration of
AtaMg. the mice were protected against audiogenic seizures after
4 h and this protection persisted for up to 72 h after the
treatment. With the other magnesium salts (PCMH and
MgCl2) maximum protection occurred by 6 h after the
injection, but after that time the number of seizures increased
sharply. Intraperitoneal taurine alone only reduced the severity
of the audiogenic seizures. The length of treatment needed to
inhibit audiogenic seizures was reduced by treatment with a
combination of vitamin B-6 (a magnesium fixing agent) and PCMH or
MgCl2. However this combination of vitamin B-6 and
magnesium salts did not prevent the recurrence of audiogenic
seizures, which was only achieved by ATaMg.
The results suggest that audiogenic seizures in
magnesium-deficient mice form a model of magnesium depletion.
This depletion is completely inhibited by the combination of an
inhibitory neurotransmitter (taurine) and magnesium, in the form
of magnesium acetyltaurinate.
Key words: Dietary
magnesium-deficient mice, magnesium depletion, magnesium
chloride, magnesium pyrrolidone-2-carboxylate, magnesium
acetyltaurine, taurine, vitamin B-6, audiogenic seizures.
Introduction
Sound stimulation of a certain frequency and range triggers
generalized convulsions (audiogenic seizures) in mice. There are
three successive stages leading to this condition:
(1) Intensive motor excitation expressed by wild
running.
(2) Clonic convulsions during which the animal falls on its side
accompanied by clonic rhythmical movement of its limbs.
(3) Tonic convulsions during which the animal stretches. During
this phase the animal may die from respiratory arrest.
Some strains of mice are naturally susceptible to audiogenic
seizures (DBA/2, Fring's O'Grady), These are called
audiosusceptible strains. Others are audioresistant (OF1, AKR,
C57BL/6) 2-4. The best known form of magnesium
deficiency in humans is expressed by neuromuscular
hyperexcitability5. Rats, which are most frequently
used in studies on experimental magnesium deficiency, may show
signs of central nervous hyperexcitability (spontaneous
convulsive seizures, audiogenic seizures) when made
magnesium-deficient. The beneficial effects of magnesium salts
(MgCl2 and MgSO4) on these central
manifestations of magnesium deficiency are well known
6-10. However, few experimental studies have been
undertaken on magnesium-deficiency-induced audiogenic seizures in
mice as a model of central hyperexcitability. Finally, the
effects of correcting magnesium deficiency by magnesium salts of
acid pyrrolidone-2-carboxylate (PCMH) and acetyltaurinate
(ATaMg), and the combination of these two molecules with vitamin
B-6, on the central manifestations of magnesium deficiency have
never been described. The aims of this study were therefore: (1)
to compare the effect of ATaMg and PCMH to that of
MgCl2; and (2) to find out whether the combination of
vitamin B-6 with magnesium salts will reduce the length of the
treatment needed to protect mice from audiogenic seizures.
Methods
Mice were made magnesium-deficient with a low magnesium diet
(50 mg of magnesium per kg of food) prepared by U.A.R. (Usine
d'Alimentation Rationnelle). Mice on control diet were fed on a
normal magnesium intake (U.A.R. dietA.03, containing about 1.70 g
of magnesium per kg of food).
Male mice of the OF1 strain from Ifacredo were placed 20 per
cage in an animal house at fixed temperature (22 ± 1°
C), under artificial lighting alternating 12 h of daylight and 12
h of darkness (from 7 am to 7 pm). The mice remained under these
conditions for at least 3 d and during this time were generously
fed a U.A.R. A..03 diet and water containing 40 mg/litre of
magnesium in chloride form. After 3 d in the cages, the animals
were fed the U.A.R. magnesium-deficient diet for a period of 40
d. This ensures a 100 per cent audiogenic seizure rate. In order
to assess the mineral intake more accurately, the mice drank only
distilled water during this period. After 40 d of this diet the
body weight per animal was 30 ± .5 g.
Audiogenic seizures were triggered by a 10 kHz ± 100
Hz), 1V intensity sinusoid signal at its highest peak, from a low
frequency Farnell-MF generator (ref. ESG/1) through a 50 x 60 x
20 cm box. Using a Tektronix RM564 spectrum analyser and a Rochar
Electronique frequency metre (ref. A1360C) as a signal check, the
signal was then amplified by a Sony TAF 300 system. The noise
level of about 100 dB was measured close to the animal's ear by
an external decibel meter probe DM 600. A time delay system
regulated the length of the sound simulation in 15 s periods.
During the audiogenic seizure test only one mouse was in the box
at a time. The number of animals completing total audiogenic
seizure cycles during each test was recorded. We used the
following compounds to correct magnesium-deficiency-induced
audiogenic seizures:
- Magnesium chloride, MgCl2.6H2O
(Prolabo reference 25 108 23)
- Magnesium pyrrolidone-2-carboxylate (PCMH) (reference MERAM
5132)
- Magnesium acetyltaurinate (ATaMg) (reference MERAM
94819)
- Sodium acetyltaurinate (ATaNa) (reference MERAM
1396/2)
- Taurine (reference MERAM 2028/4)
We used the Shapiro Wilk test to check for normal distribution
among the groups. The Kruskall Wallis test, an analysis of
non-parametric variances, was used to compare the different
groups.
Results
Correction of audiogenic seizure with
magnesium salts
Magnesium intake through an oesophageal probe
For this experiment 40 batches of 20 male OF1 mice were fed a
diet deficient in magnesium for 40 d. At the end of this period
we checked all the mice in two of the batches of the control
animals for audiogenic seizure susceptibility. Later, these two
batches of control mice were fed a diet with normal magnesium
content, i.e., about 1.7 g of magnesium per kg of food
(UARA 03 diet) and 40 mg/litre of water intake (magnesium
chloride in drinking water) and for 10 d they were fed distilled
water instead of magnesium salts.
The other batches of mice were fed their normal diet with a
magnesium supplement (3,5,7,14 or 28 mg) in the form of different
magnesium salts. For each type of magnesium salt administered
(ATaMg, PCMH, MgCl2) we used three batches of mice per
product and per dose.
The product was given in the morning and the audiogenic tests
were carried out the next day around 3 pm. Once the treatment was
given, the animals were kept under surveillance (a diet of 40
mg/litre with a normal content of magnesium in drinking water)
and were once more subjected to audiogenic tests 24, 31, 38, 45,
52 and 60 d later.
Even after 10 d of treatment with different salts and a
magnesium supplement of 3.5 mg or 7 mg/d all the animals were not
totally protected from audiogenic seizures. On the other hand, an
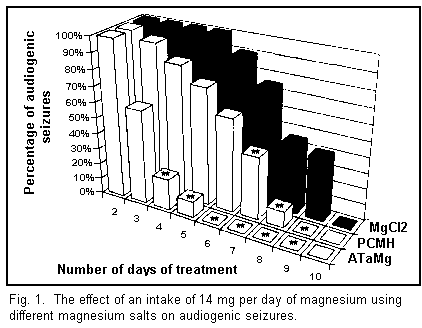
intake of 14 mg/d of magnesium (see Fig. 1) in its different
forms helps to protect all the animals from audiogenic seizures
after following diets which vary according to the type of salts
used. The most effective molecule used was ATaMg, since it took
only 6 days of treatment to totally eliminate audiogenic
convulsions among all the mice. It is noteworthy that a daily
intake of 28 mg of the various magnesium salts brought about no
significant change in the length of the treatment required to
terminate all audiogenic seizures.
At the end of the treatment the animals were again subjected
to audiogenic seizure tests once a week from the 24th day. The
results obtained using PCMH and MgCl2 (Fig. 2) show
that a certain percentage of the animals which were fed these
compounds did have audiogenic seizures. These results are similar
to those obtained in the magnesium-deficient animals and in those
reestablished on normal diet. On the other hand, the animals
treated with ATaMg were definitely protected from audiogenic
seizures.
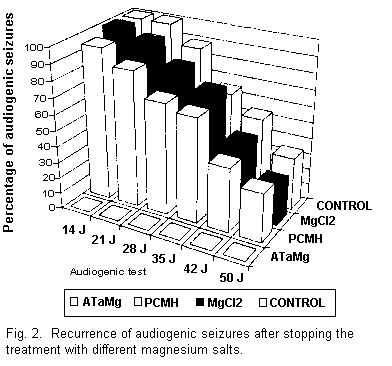
After 31 d on a normal diet audiogenic seizures were observed
in all the control mice. The normal diet had to be continued for
at least 52 d in order to protect the other half of the
batch.
Finally, it was observed that at the end of the experiment,
i.e., on the 60th day, one third of the control animals
were still susceptible to audiogenic seizures.
Magnesium salts or taurine by parenteral
injection
For this test we used three batches of 10 male OF1 mice after
a 40 d magnesium deficiency period. The different compounds were
given intraperitoneally at 24 h intervals (from day 1 to day 3).
The audiogenic test was carried out 2, 4, 6, 24, 48 and 72 h
after the last injection. Injections of magnesium (3 mmol/kg)
were given in the form of ATaMg (1070 mg/kg), PCMH (986 mg/kg)
and MgCl2 (613 mg/kg). A dose of 1200 mg/kg of taurine
was injected. During this experiment we observed the different
stages of the audiogenic seizure cycle (wild running, clonic
convulsions, tonic convulsions and fatal convulsions). The
results are illustrated in Figs 3 to 5.
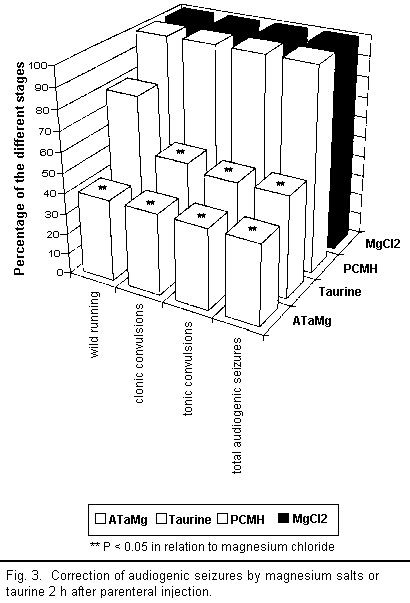
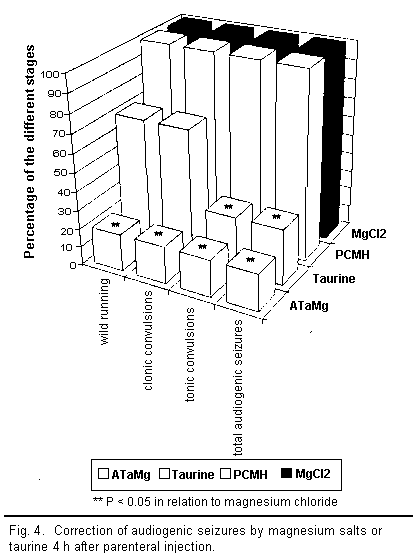
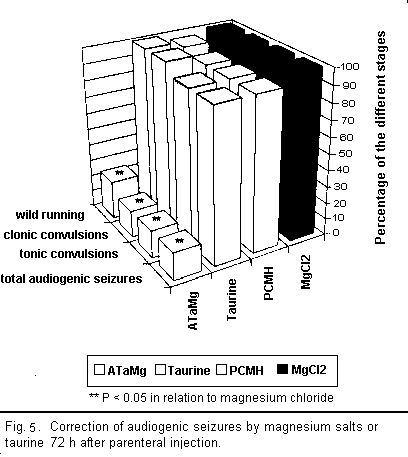
A qualitative analysis of the results shows that all the
compounds injected intraperitoneally (ATaMg, PCMH,
MgCl2, and taurine) helped to diminish
magnesium-deficiency-induced audiogenic seizures more or less
efficiently depending on the compound used. However, there were
major differences in quality between compounds.
Two hours after injecting ATaMg, effective protection from
audiogenic seizures was observed and persisted for 72 h after the
treatment, reaching its maximum level about 4 h after injection.
Protection was equally effective during all stages of the
audiogenic seizure cycle (particularly in the reduction of the
wild running phase). The maximum effect of magnesium chloride and
PCMH on audiogenic seizures appeared 6 h after injection but
remained well below that of ATaMg. Here too there was equal
protection from audiogenic seizure during all the different
stages; however, after 24 h, this protection ceased to be
effective.
Taurine or magnesium acetyltaurinate have similar effects on
magnesium-deficiency-induced audiogenic seizures but only the
results for taurine are presented in the figures. The action of
taurine or sodium acetyltaurinate on magnesium-deficiency-induced
audiogenic seizures is complex and, in contrast to the other
compounds used, did not equally affect all stages of the
convulsions. Indeed, 2 h after injection there was a decline in
the number of clonic and tonic convulsions, whereas the wild
running did not diminish significantly in any way. Four hours
after the injection, the number of clonic convulsions increased
and equaled the number of wild running movements. These two
stages of audiogenic seizure develop along the same lines and
affected 100 per cent of the mice from 24 h onward. The number of
tonic convulsions decreased after 2 h and remained low up to 48 h
before increasing again. Thus it seems that the action of taurine
or sodium acetyltaurinate is characterised only by the
non-occurrence of tonic convulsions among a large number of the
mice.
Correction of audiogenic seizures through the
combination of magnesium salts and vitamin B-6
The role of vitamin B-6 as a magnesium fixing compound has
been fully described and used in therapy. We sought to determine
whether the combination of vitamin B-6 with magnesium salts helps
to shorten the duration of the treatment required in order to
protect our model animal with neuromuscular hyperexcitability
from audiogenic seizure.
For this test we used 40 d magnesium-deficient OF1 mice (each
weighing about 30-33 g). The mice were in batches of 20. At the
end of the magnesium deficiency period the animals were fed a
diet with a normal magnesium content and separated into four
batches. The first batch was fed 30 mg/kg per day of vitamin B-6
alone for 10 d through an oesophageal probe. The other batches
were fed the different magnesium salts and 30 mg/kg per day of
vitamin B-6 for 10 d. During the treatment period all the animals
were submitted to audiogenic seizure tests on a daily basis. At
the end of the treatment period, the animals were once more
submitted to audiogenic seizure tests 24, 31, 38, 45, 52 and 60 d
later.
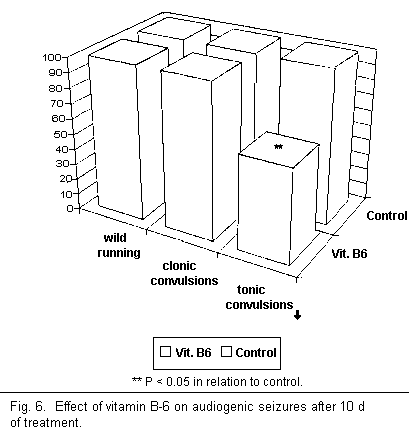
After 10 d of treatment complete audiogenic seizure frequency
was reduced by 50 per cent through the use of vitamin B-6 alone
(see Fig. 6). However, these results show that use of vitamin B-6
does not ensure complete protection against audiogenic seizures,
since it only lowered the number of tonic convulsions in the
control mice significantly and had no effect on the first two
stages of the audiogenic seizure cycle (wild running and clonic
convulsions). Thus rather than ensuring guaranteed protection
from audiogenic seizures there was in fact only an attenuation of
the cycle.
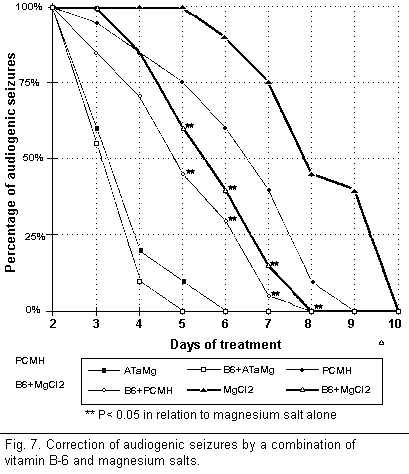
On the other hand, the combination of vitamin B-6 and
magnesium salts (Fig. 7) helped to protect the mice from
audiogenic seizures by interacting during all stages of the
cycle. A comparison of the percentage of audiogenic seizures
obtained when combining each type of salt with vitamin B-6 shows
that during treatment for a chosen period of time the percentage
was significantly lower when using PCMH and MgCl2, and
only slightly lower when using AtaMg. However, the combination of
vitamin B-6 and MgCl2 or PCMH was far less effective
than ATaMg alone or ATaMg in combination with vitamin B-6.
Further at the end of the treatment audiogenic seizures recurred
only among those mice treated with vitamin B-6 alone or with B-6
in combination with MgCl2 or PCMH. Those animals
treated with vitamin B-6 and ATaMg were permanently protected
from audiogenic seizures.
Discussion
Oral magnesium intake
On the basis of quality the correcting effect of the three
types of magnesium salts was the same. In fact, pharmacological
doses of magnesium (14 mg per day per mouse or about 500 mg/kg
per day) suppresses all magnesium-deficiency-induced audiogenic
seizure activity. This adds to a previous study11
which shows that MgCl2 suppresses seizures among OF1
mice made magnesium-deficient for 43 days, and confirms other
results which show that MgCl2 or MgSO4,
when given in pharmacological doses, are capable of suppressing
convulsive seizures in the rat 6,7,12 and
mouse8,13,14. On the other hand, when
magnesium-deficient animals are again fed a normal diet,
audiogenic seizures recur. Thus the nature of the deficit is not
simply a case of magnesium deficiency but one of magnesium
depletion.
In terms of quantity, the correcting effect of the three salts
is different. Indeed, processing the results statistically using
the Kruskall-Wallis test shows that the action of ATaMg or PCMH
is greater than that of MgCl2 in inhibiting audiogenic
seizures. Moreover, ATaMg is the more effective salt since, for
each equal dose of magnesium administered, this compound
suppresses the seizures more quickly than the other salts. This
salt is seen to be most effective even when providing a low
intake of magnesium (3.5 mg/kg per day of Mg2+), at
which level one third of the animals were protected from
audiogenic seizures.
The fact that PCMH is more effective in suppressing audiogenic
seizures than MgCl2 was expected since it is well
knownl5 that among mice that are not
magnesium-deficient the intracellular penetration of magnesium is
more active with PCMH than with MgCl2. This may well
be due to the ion pyrrolidine-2-carboxylate-5, which sensitizes
the cellular receptors for the cation Mg2+.
Furthermore, other investigators 16 have shown that
administering PCMH to magnesium deficient animals produces an
increase in the magnesium level equal to that of the magnesium
fed control animals after 10 days of treatment, whereas
administering MgCl2 is not followed by a return to
normal levels of magnesium under the same conditions. However,
the difference between these two salts is most striking with
regard to skin problems, which heal more quickly and more
completely with PCMH than with MgCl2. Thus our
research is complementary to these results in showing that PCMH
is more effective than magnesium chloride in treating central
nervous system problems associated with severe magnesium
deficiency.
Magnesium load by parenteral injection
We showed that ATaMg was the most effective compound when
given either by injection or orally in protecting
magnesium-deficient mice from audiogenic seizures. Indeed, ATaMg
injection equivalent to a dose of 3 mmol/kg of magnesium ensured
protection throughout all stages of audiogenic seizure from the
second hour after the injection. The effect reached its maximum
level 4h after the injection and persisted for 72 h. In contrast,
injecting the other salts caused a reduction in the number of
audiogenic seizures after 6 h, but after 24 h convulsions
recurred in all the animals.
That ATaMg should be extremely effective was foreseeable,
since taurine is known to have a positive effect on some types of
convulsion17,18 (hyperbaric oxygen, cobalt injections)
in the rat and the mouse. Furthermore, studies by Batuev et
al. 19 have shown that three intraperitoneal
injections of taurine stop the convulsions accompanying
audiogenic seizures in genetically audiosensitive rats. This is
why the fact that ATaMg is more effective than the other
magnesium salts (when expressed in equal doses of magnesium) is
undoubtedly linked to the taurinergic component in the molecule
rather than to the magnesium. In this case, the greater
effectiveness of ATaMg may be due to the following:
(1) A purely anticonvulsive action in the taurinergic component
of the molecule. However, it is noteworthy that taurine does not
have the capacity to suppress all stages of audiogenic seizure in
genetically audiosensitive rats19, but only interacts
on the severity of the seizure.
(2) The action of taurine on the cellular effects of magnesium
deficiency. Indeed, taurine is released in magnesium-deficient
rats20 and this is undoubtedly a major compensatory
mechanism which restrains the central nervous hyperexcitability
of the animal. It can be assumed that the action of the ATaMg is
due to an increase in the taurine levels in the brain blocking
the intracellular Ca2+, which in so doing increases
the rapport AMPc/GMPc21 and induces hyperpolarization
of the cell. Under these conditions, central nervous
hyperexcitability is contained.
Combination of magnesium salts with vitamin
B-6
In therapy5 it is known that the combination of
vitamin B-6 and magnesium is beneficial in the treatment of
neuromuscular forms of primary magnesium deficiency. Studies by
Majumdar & Boylan22 show clearly that a diet
supplement of vitamin B-6 administered to a magnesium-deficient
rat increases the magnesium level in its different tissues and in
particular, in brain tissue. Furthermore, this increase is in
relation to the dose of vitamin B-6 given to the animal. Although
the precise mechanism of the interaction between vitamin B-6 and
magnesium is not completely known, it seems an established
fact5,23 that vitamin B-6 speeds up the transport of
magnesium through the cellular membranes and is essential for
storing magnesium. In vitro it has been
established24 that magnesium may form a coordinated
compound with pyridoxal phosphate, but the physiological role of
this compound is as yet unknown.
The combination of vitamin B-6 with PCMH or MgCl2
helps reduce the length of the treatment period required to
protect all the magnesium-deficient mice from developing
audiogenic seizures. This reduced treatment period is probably
due to a much more active transport of magnesium to the brain.
This may more rapidly restore the metabolic processes disrupted
by magnesium deficiency and which are responsible for inducing
audiogenic seizures. This seems to be the case of the combination
of vitamin B-6 and magnesium salts (PCMH and MgCl2).
Vitamin B-6 alone does not ensure total protection as it only
reduces the severity of the audiogenic seizures (reduction in
tonic convulsions) of magnesium-deficient mice. This confirmed by
the studies of Schlesinger & Boggan25 and
Schlesinger & Lieff26 which show that vitamin B-6
acts only on the severity of the audiogenic seizures by reducing
the number of fatal convulsions.
Paradoxically, a comparison of our results obtained from
combining vitamin B-6 with ATaMg to those observed with ATaMg
alone does not show any major reduction in the length of
treatment required to protect all the magnesium-deficient-mice
from audiogenic seizures. There are two possible reasons for
this:
(1) Vitamin B-6 does not form a compound with the magnesium
contained in the AtaMg. A study is under way to determine the
complexation constants between these different molecules and
magnesium.
(2) The taurinergic component of the molecule is greater than the
magnesium component when used to correct audiogenic seizures.
Thus it can be assumed that the action of ATaMg in correcting
audiogenic seizures is different from that of PCMH and
MgCl2. It is conceivable that the combination of
vitamin B-6 and ATaMg brings about no change.
Finally, the combination of vitamin B-6 and magnesium salts
(except ATaMg) did not stop the recurrence of audiogenic
seizures. Thus it is conceivable that audiogenic seizures are a
sign of magnesium depletion in which the combination of magnesium
salts with vitamin B-6 brings about no change.
Conclusion
The basis of any treatment for magnesium deficiency consists
of oral administration of physiological doses (5 mg/kg per day)
of a magnesium salt5. In this model of audiogenic
seizures in magnesium-deficient mice, (1) pharmacological intakes
(about 500 mg/kg per day) of magnesium salts alone combined with
a normal dietary intake of magnesium were able to stop all
audiogenic seizures; (2) these convulsive seizures recurred on
discontinuing treatment. Increasing the length of the treatment
period using PCMH or magnesium chloride did not always prevent
the recurrence of audiogenic seizures. Finally, vitamin B-6 used
as a magnesium fixing compound did not protect the animals
permanently from audiogenic seizures.
These unsuccessful results lead us to the conclusion that
audiogenic seizures in magnesium-deficient mice are not due
simply to magnesium deficiency but rather to magnesium depletion
5. Parenteral injections of MgCl2 or PCMH
do not protect the animals permanently from audiogenic seizures,
even after 7-15 days of treatment (unpublished results). It can
be assumed that the principal mechanism of the depleted animal
model is in no way linked to magnesium deficiency. On the other
hand, the fact that ATaMg administered orally or by
intraperitoneal injection protects the animal permanently from
audiogenic seizures leads us to the conclusion that taurine acts
in a very significant way on the magnesium-depleted animal model.
However, it is noteworthy that taurine or sodium acetyltaurinate
do not have a lasting beneficial effect on audiogenic seizures.
It seems that the magnesium-depleted animal model is highly
sensitive to the combination of an inhibiting neurotransmitter
with a taurine component and magnesium in the same molecule.
Acknowledgements
We thank Mr J. Harper for his translation.
References
1. Frings, H.M., Fuller, J.L., Ginsburg, B.E., Ross S. &
Vicari, E.M. (1952): Standardization of nomenclature describing
audiogenic seizures in mice. Behavior 4, 157-160.
2. Seyfried, T.N. & Glaser, G.H. (1985): A review of mouse
mutants as genetic models of epilepsy. Epilepsia 26, 143-150.
3. Fuller, J.L., Easier, C. & Smith, M.E. (1950):
Inheritance of audiogenic seizure susceptibility in the mouse.
Genetics 35, 622-632.
4. Fuller, J.L. & Sjursen, F.H.Jr. (1967): Audiogenic
seizures in eleven mouse strains. J. Hered. 58, 135-140.
5. Durlach, J. (1988): Magnesium in clinical practice. London:
John Libbey.
6. Chutkow, J.G. (1974): Clinical-chemical correlation in the
encephalopathy of magnesium deficiency. Effect of reversal of
magnesium deficits. Mayo Clin. Proc. 49, 244-247.
7. Chutkow. J.G. (1974): Metabolism of magnesium in central
nervous system: relationship between concentrations of magnesium
in cerebrospinal fluid and brain in magnesium deficiency.
Neurology 24, 780-787.
8. Buck, D.R., Mahoney, A.W. & Hendricks, D.G. (1976):
Effect of magnesium deficiency on Nonspecific Excitability Level
(NEL) and audiogenic seizure susceptibility. Pharmacol. Biochem.
Behav. 5, 529-534.
9. Poenaru, S., Durlach, J., Rouhant, S., Rayssiguier, Y.,
Gueux, E., Iovino, M. & et Reba, A., (1983): Etude
électrophysiologique de la carence magnésique du
rat. Magnésium 2, 299-312.
10. Poenaru, S., Rouhani, S., Gueux, E., Opriou, A., Aymard,
N. et Iovino, M. (1983): Tétanie
hypomagnésémique expérimentale
traitée: étude électrophysiologique.
Magnesium Bull. 2, 47-52.
11. Bac, P. (1981): Crise audiogène chez la souris
selon la souche et le sexe. Influence de la ration
magnésique et des neuromédiateurs. Reprod. Nutr.
Devel. 21, 3, 429-440.
12. Chutkow, J. G. & Meyers, S. (1968): Chemical changes
in the cerebrospinal fluid and brain in magnesium deficiency.
Neurology 18, 963-974.
13. Belknap, J.K., Berg, J.H., Cocke, R. & Clancy, A.N.
(1977): Induction and reversal of the magnesium deficiency
syndrome in inbred mice. Exp. Neurol. 57, 506-515.
14. Belknap, J.K., Berg, J.H., Ondrusek, G. & Waddingham,
S. (1978): Barbiturate withdrawal and magnesium deficiency in
mice. Psychopharmacology 59, 299-303.
15. Binet, P., Miocque, M. Pechery, C., Roux, M. &
Rinjard, P. (1976): Etude expérimentale de quelques
propriétés pharmacodynamiques du
pyrrolidone-2-carboxylate-5 de magnésium. Thérapie
31, 47 l-481.
16. Binet, P., Miocque, M.. Pechery, C.. Roux, M. &
Rinjard, P. (1978): Effet correcteur comparé du
pyrrolidone-2 carboxylate de magnésium et du chlorure de
magnésium sur les troubles cutanés et
hématologiques de la carence magnésique
expérimentale due Rat. Thérapie 33, 491-500.
17. Adembri. G., Bartolini, R., Giotti, A. & Zilleti, L.
(1974):Anticonvulsive action of homotaurine and taurine. Br. J.
Pharmacol. 52, 439-440.
18. Craig, C.R. (1984): Evidence for a role of
neurotransmitters in the mechanism of topical convulsant models.
Fed. Proc. 43, 2525-2528.
19. Batuev, A.S., Ryabinskaya, E.A. & Gudimova, N.V.
(1979): L'action de la taurine sur les accès convulsifs
audiogènes chez les rats (in Russian). Dokl. Akad. Nauk.
SSSR 248, 1496-1499.
20. Durlach. J., Poenaru, J., Rouhani, S., Bara, M. &
Guiet-Bara, A. (1987): The control of central neural
hyperexcitability in magnesium deficiency. In: Nutrients and
brain function, ed. W.B. Essman, pp. 49-71. Basel: Karger.
21. Rapin, J.P,, Galiez, V., Le Poncin-Lafitte, M., Durlach,
J., Rayssiguier, Y. et Godard, J.P. (1983): Distribution
régionale des nucléotides cycliques dans le cerveau
de rats adultes carenccés en magnésium.
Magnesium-Bull. 2, 87-91.
22. Majumdar, P. & Boylan, L.M. (1989): Alteration of
tissue magnesium levels in rats by dietary vitamin B6
supplementation. Int. J. Vitam. Nutr. Res. 59, 300-303.
23. Durlach, J. (1969): Données actuelles sur les
mécanismes de synergie entre vitamine B6 et
magnésium. J. Méd. Besançon 5, 349-359.
24. Boylan, L.M. & Spallholz. J.E. (1990): in
vitro evidence for a relationship between magnesium and
vitamin B6. Magnesium Res. 3, 79-85.
25. Schlesinger, K. & Boggan, W. (1968): Genetics of
audiogenic seizures: II-Effects of pharmacological manipulation
of brain serotonin, norepinephrine and gamma aminobutyric acid.
Life Sci. 7, 437-447.
26. Schlesinger, K. & Lieff, B. (1975): Levels of
pyridoxine and susceptibility to electroconvulsive and audiogenic
seizures. Psychopharmacologia 42, 27-32.
All articles by Dr. Durlach are copyrighted, and permission is
granted to Web users only to make single hard copies for personal
use. Additional reprints should be obtained from the originating
journals. Excerpts may be used by the media with attribution to
Dr. Durlach.
This page was first uploaded to The Magnesium Web Site on
April 19, 1996
http://www.mgwater.com/







