ORIGINAL ARTICLE
Magnesium Research (1989) 2, 4, 243-247
A qualitative theory of the screening-binding effects of
magnesium salts on epithelial cell membranes: a new
hypothesis
M. Bara1, A. Guiet-Bara2, J.
Durlach3
1Laboratory of Biology of Reproduction, University
P. M. Curie, 7 Quai Saint-Bernard, 75252 Paris Cedex 05;
2Laboratory of Biology of Reproduction, University P.
M. Curie, 7 Quai Saint-Bernard, 75252 Paris Cedex 05;
3CHU Cochin Port-Royal, 27 Rue du Faubourg
Saint-Jacques, 75674 Paris Cedex 14, France
Summary: A theoretical explanation
is given of the screening-binding effects of various magnesium
salts on the ionic permeability of epithelial amniotic cell
membranes. It is suggested that the 'screening process' induces
an increase in the electrical membrane resistance and in membrane
stability which is a unique action at low concentration. At high
concentration, the binding process induces a reduction or an
increase in these parameters as a function of the magnesium salt
present. The different effects are due to changes in the
distribution and in the repartition of the fixed charges on the
cell membrane.
Key words: Binding, magnesium,
membrane, screening.
Introduction
There are two categories of electrostatic interactions between
cations and negatively charged surfaces 1,2. In the
first category, there is the usual type of electrostatic binding
where the cations complex to anionic surface moities and are not
mobile. In the second category of associations, often referred to
as 'screening', the cations remain mobile, most likely fully
hydrated and unbound, being held loosely by hydrogen bonds in a
diffuse layer close to the surface 3. The two types of
interactions together can explain the effects of cations on the
stability of the membrane.
In studies on ionic permeability through the human isolated
amniotic membrane, it has been shown 1,4 that
different magnesium salts have different effects on the membrane
stability, as follows (Table):
| Table. The magnesium salts
employed. |
|
| Magnesium salt |
| hydrated |
Name |
|
| MgSO4.7H2O |
Magnesium sulphate heptahydrate |
| Mg(Cl2.6H2O |
Magnesium chloride hexahydrate |
| Mg (NO3)2.2H2O |
Magnesium nitrate dihydrate |
| Mg
(CH3COO)2.4H2O |
Magnesium acetate tetrahydrate |
| Mg
(C3H3O3)2.3H2O |
Magnesium lactate trihydrate |
|
Mg(HC6H5O7).5H2O |
Magnesium citrate pentahydrate |
|
1. The salts MgCl.6H2O,
Mg(CH3OO)2.4H2O, and magnesium
citrate increase the stability at low concentrations, then
decrease it at higher concentrations on both sides of the
amnion.
2. The effects of the salts of MgSO4 and
Mg(NO3)2.2H2O are independent of
concentration. They increase the stability on the maternal side
and decrease it on the fetal side.
3. The salt of magnesium lactate increases the stability on
the maternal side, whatever the concentration; it increases the
stability on the fetal side at low concentrations, and it
decreases it at high concentrations.
We now propose a theory to explain the effects of the addition
of magnesium salts on the surface polar groups, which are
responsible for the exchange through the isolated human
amnion.
Hypothesis
This new theory concerns the 'screening' and binding
effects.
The 'screening effect' (Figure 1)
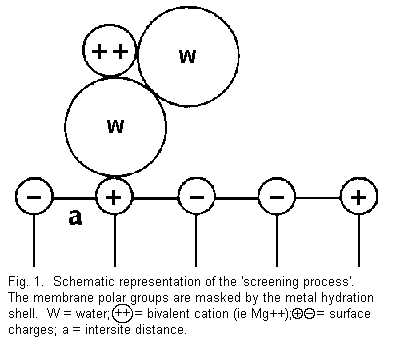
The term 'screening' is used to show the existence of ions at
a distance of a few angstroms from the membrane surface in the
aqueous diffuse layer and to distinguish this effect from the
phenomenon of the specific adsorption 5 . In this
case, the distance membrane polar groups (positive and negative),
whatever their location and distribution, are masked by the
hydration shell around the cations and that this reduces
considerably the repulsive or attractive forces between the
divalent cations and the surface charges, but there are now
H-bond interactions 3. The external sites on the
membrane are not accessible to the ions present in the medium and
the electrical resistance of the membrane increases; thus the
'screening process' induces an increase of the membrane
stability.
The binding effect (Figures 2-5)
In the binding processes, the cations have lost their
hydration shell and the interactions between cations and surface
polar groups are direct and possible. These interactions are a
function of the location and the distribution of the surface
binding sites. In this work, we have analysed different
situations depending to the location of the external sites, which
are illustrated in Figs 2-5.
In Fig. 2 it can be seen that:
1. the divalent cations are near the surface sites
and there are attractive forces between cations and negative
surface sites (L-) and repulsive forces between cations and
positive surface sites (L+);
2. the divalent cations are drawn towards two
negative sites (L-) with formation of strong covalent bonds
between cation (Mg2+) and sites; and
3. there is formation of neutral complexes of the
type [L-Mg-L]0 (zero charged magnesium complexes)--the
number of accessible sites is reduced, the electrical membrane
resistance increases and the membrane stability is increased.
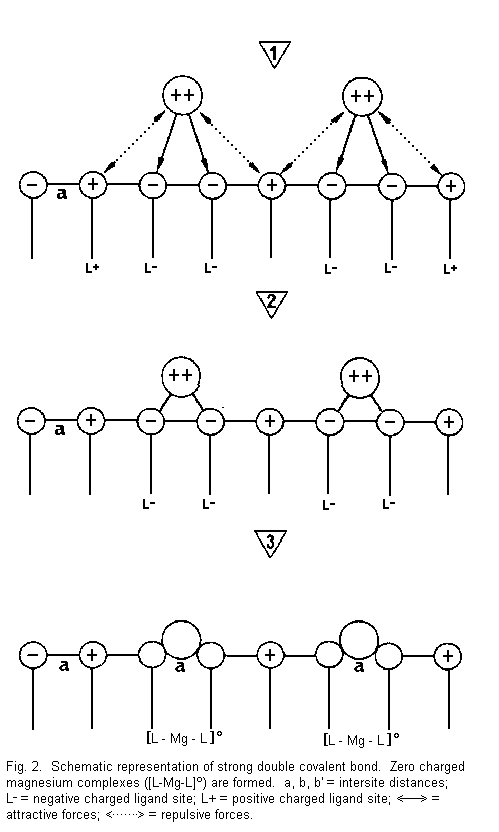
Figure 3 shows that:
(1) the divalent cations are again near the surface
sites and there are attractive forces between cations and
negative surface sites (L-), and repulsive forces between cations
and positive surface sites (L+);
(2) the divalent cations are drawn towards one
negative side (L-) with formation of a strong covalent bond and
repulsion with the positive surface sites (L+); and
(3) there is neutralization of a negative surface
site by one positive divalent cation charge. There is formation
of positive complexes of the type [Mg-L]+ (singly
charged magnesium complexes). A positive surface site is
established instead of a negative surface charge. A repulsion
with regard to an adjacent positive site results, the binding
surface sites is weakened, and the intersite distance is
increased (b>a) -- the electrical membrane resistance is
reduced and the membrane stability is decreased.
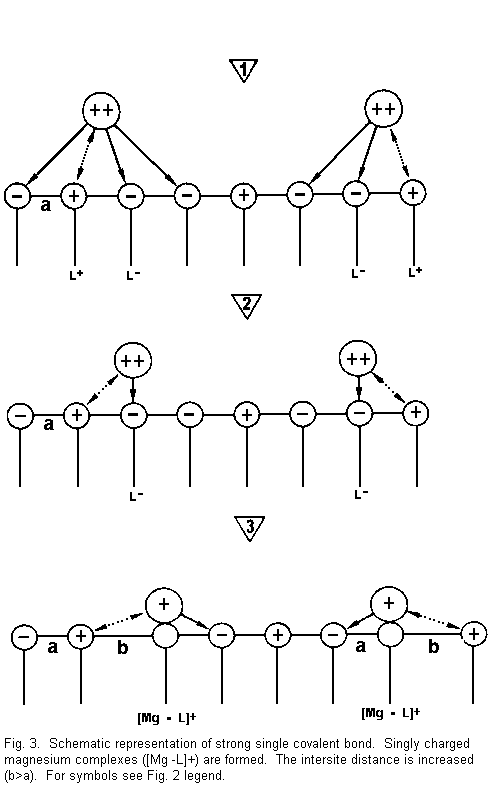
Figure 4 shows that:
1. the divalent cations are again near the surface
sites and there are attractive forces between cations and
negative surface sites (L-), and repulsive forces between cations
and positive surface sites (L+);
2. the divalent cations are drawn towards one
negative site (L-) with formation of a strong covalent bond and
repulsion of the positive surface sites; and
3. there is neutralization of a negative surface
site by one positive divalent cation charge. There is formation
of positive complexes of the type [Mg-L]+ singly
charged magnesium complexes. A positive surface site is
established instead of a negative surface charge. A repulsion
with regard to adjacent positive sites is induced, the bindings
between surface sites are weakened, and the intersite distance is
increased (b>a and b'<a); as a result the electrical
membrane resistance is reduced and the membrane stability is
decreased.
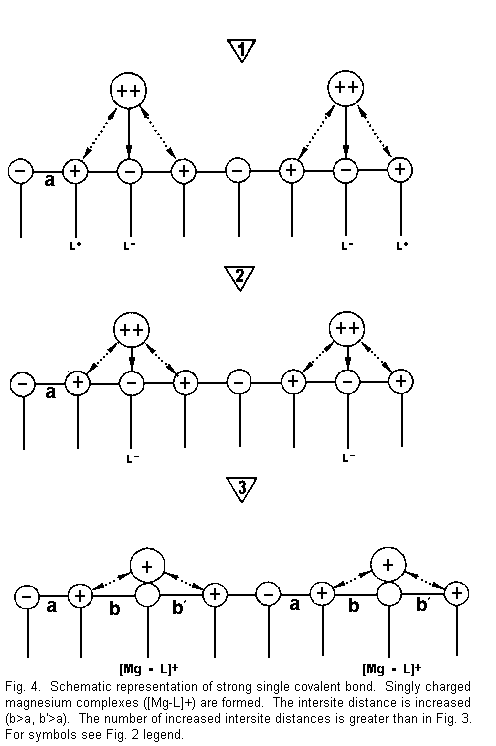
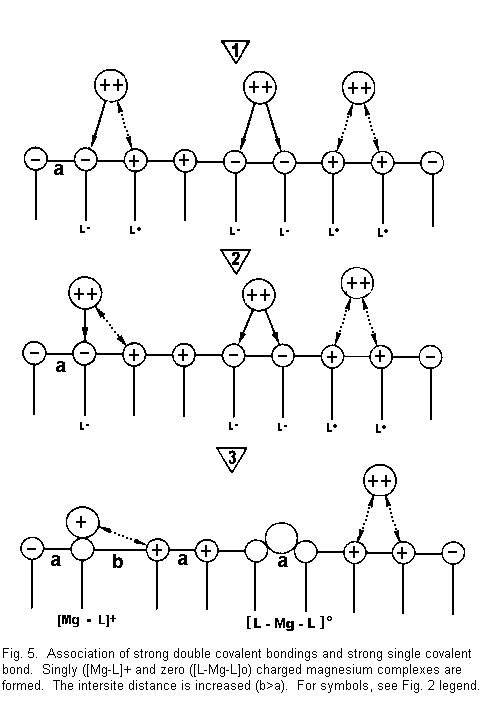
Figure 5 shows that:
1. the divalent cations are again near the surface
sites and there are attractive forces between cations and
negative surface sites (L-), and repulsive forces between cations
and positive surface sites (L+);
2. in this case, some (divalent cations are drawn
towards two negative sites (L-) with formation of strong covalent
bonds, some other divalent cations are drawn towards one negative
site (L-) and repel the adjacent positive site (L+), and some
repel two adjacent positive sites; and
3. there are three possible situations:
- (a) there is neutralization of a negative surface site by
one positive divalent cation charge. There is formation of
positive complexes of the type [Mg-L]+ singly
charged magnesium complexes. A positive surface site is
established instead of a negative surface site. A repulsion
with regard to an adjacent positive site is induced and the
bonds between surface sites are weakened. The intersite
distance is increased (b>a), the electrical membrane
resistance is reduced, and the membrane stability is decreased
(Fig. 5 left);
- (b) there is formation of neutral complexes of the type
[L-Mg-L]0. The number of accessible sites is
reduced, the electrical membrane resistance increases, and the
membrane stability is increased (Fig. 5, middle);
- (c) Some divalent cations are not fixed on the sites
(repulsive forces) but they mask these sites with their
hydration shell for other ions; the electrical membrane
resistance increases and the membrane stability is increased
(Fig. 5, right).
The result of the cases described in Fig. 5 may be either a
decrease (left) or an increase (middle and right) in the membrane
stability.
The distribution and the location of the surface sites will
determine the binding process which may be of two different
forms:
(1) an increase in the membrane stability (Figs 2,
and 5 middle and right);
(2) a decrease of the membrane stability (Figs 3, 4
and 5 left).
Implications
The results obtained with various magnesium salts on the ionic
permeability of the human amnion 1,4, may be explained
by the above theoretical considerations.
Maternal side
At low concentrations, all magnesium salts decrease the
electrical conductance and the ionic fluxes. This action may be
described as a 'screening effect' (Fig. 1): the hydration shell
is present, the salts are highly hydrated and the external sites
are masked. The electrical membrane resistance increases
1,4.
At high concentrations, it has been shown that there are two
effects as a function of the magnesium salt present.
(a) A 'screening effect' (concerning, ie
MgSO4, Mg lactate, Mg(NO3)2) has been
described because of a decrease in the electrical conductance or
the ionic fluxes 1,4 . In this case, the hydration
shell is still present, but in the preceding hypothesis the
interaction may be more complex: a decrease in the electrical
conductance and in the ionic fluxes may be observed in a binding
saturation (Figs 2 and 5, middle and right). This explanation may
be applicable to the action of these magnesium salts at high
concentration.
(b) A binding effect (concerning, ie,
MgCl2, Mg acetate, Mg citrate) has been described,
because of an increase in the electrical conductance and the
ionic fluxes 1,4. These observations correspond to the
situation described in Figs 3, 4 and 5 (left).
Fetal side
At low concentrations, the electrical conductance and the
ionic fluxes are decreased by all the magnesium salts studied
except MgSO4 and Mg(NO3)2 1,4.
In general, there is a 'screening effect' (Fig. 1). However, with
MgSO4, and Mg nitrate the hydration shell is
immediately lost, in favour of a strong attraction between these
salts and the external surface sites of the fetal face. This
effect may be related to the lipid composition of the fetal
membrane 3,6.
At high concentrations, the electrical conductance and the
ionic fluxes are increased by all magnesium salts. This
corresponds to the binding effect described in Figs 3, 4 and 5
(left).
Discussion
It has often been suggested that fixed electrical charges in
the cell membrane are related to the ionic mechanism and
electrical activity of the cell. Thus, fixed charges have been
implicated in hypotheses related to the ionic transport across
the membrane 7,8.
Our results 1,4 have been analysed in terms of
changes in surface potential by 'screening' of counter-ions in
the external solution which form a diffuse double layer at the
surface, or by the binding of cations to the fixed charges on the
cell membranes. If the negative charge density on the membrane is
high, the diffuse double layer theory predicts that the addition
of even a low concentration of divalent cations to one side of
the membrane will reduce the negative surface potential on that
side by a 'screening process'. The increase in the negative
surface potential would be the result of a binding process. The
magnesium salts interact with the surface charges of the
epithelial amniotic cell by 'screening' and binding processes.
The 'screening process' induces an increase in tile electrical
resistance and the membrane stability.
The new hypothesis shows that the binding process induces
either a decrease or an increase in the electrical resistance and
the membrane stability. The induction of these two processes is
generally due to the concentration of the magnesium salts . In a
binding process, the increase or decrease in the membrane
stability is a function of the distribution and the location of
the external sites. These two parameters are not fixed in the
membrane and are conditioned by the external medium. The
magnesium salts affect the packing of the phospholipids in a
membrane and can lead to membrane alterations, such as
morphological changes or site distribution changes. One might
hypothesize that the disturbance due to the insertion of a few
molecules between the lipid layers affects the membrane
sufficiently to change its binding characteristics
9.
Conclusion
The various effects of magnesium salts on the membrane
stability may be explained with this hypothesis, which shows the
important role of the anion. Indeed, with a common cation
(Mg2+), different effects may be obtained after anion
change. Tile surface charges react differently and/or selectivity
with the membrane, and its stability may be increased or
decreased. Furthermore, at high salt concentrations, the
hydration shell may disappear and a direct binding process may
result as a function of the distribution, repartition of fixed
charges, and anions present: the membrane stability may increase
or decrease.
References:
1. Bara, M., Guiet-Bara, A. & Durlach, J. (1988): Analysis
of magnesium membraneous effects: binding and screening.
Magnesium Res. 1, 29-33.
2. Puskin, J.S.. (1977): Divalent cation binding to
phospholipids: an EPR study. J. Memb. Biol.
35, 39-55.
3. Theophanides, T., Angiboust, J.F., Anastassopoulou, J.
& Manfait, M. (1990): Possible role of water structure in
magnesium biological Systems. Magnesium Res. (in
press).
4. Bara, M., Guiet-Bara, A. & Durlach, J. (1988):
Modification of human amniotic membrane stability after addition
of magnesium salts.. Magnesium Res. 1,
23-28.
5. Kass, R.S. & Krafte, D.S. (1987): Negative surface
charge density near heart calcium channels. J. Geit.
Physiol. 89, 629-644.
6. Bara, M. (1982): Electron transport through human
epithelial amniotic membrane in vitro. Comparative study
with artificial membranes (bilayer and millipore filters).
Bioelectr. Bioenerg. 9, 517-525.
7. Teorell, T. (1935): An attempt to formulate a quantitative
theory of membrane permeability. Proc. Soc. Exp.Biol.
Med. 33, 282-285.
8. Ling, G.N. (1952): The role of phosphorus in the
maintenance of the resting potential and selective accumulation
in frog muscle cells. In Phosphorus metabolism, ed W.D.
McElroy & B. Glass, vol. 2. Baltimore: Johns Hopkins
Press.
9. Zachowski, A. & Durand, P. (1988): Biphasic nature of
the binding of cationic amphipaths with artificial and biological
membranes. Biochem. Biophys. Acta 937,
411-416.
All articles by Dr. Durlach are copyrighted, and permission is
granted to Web users only to make single hard copies for personal
use. Additional reprints should be obtained from the originating
journals. Excerpts may be used by the media with attribution to
Dr. Durlach.
This page was first uploaded to The Magnesium Web Site on
April 29, 1996
http://www.mgwater.com/





