Magnesium in clinical practice
Jean DURLACH
Translated by David WILSON
French edition (Le magnesium en pratique cliniques. Published
in 1985 by J. B. Baillière, Editions Médicales
Internationales, 62 rue des Mathurins, 75008 Paris, France
First English language edition published in 1988 by
John Libbey & Company Ltd, 80/84 Bondway,
London SW8 1SF, England (01) 582 5266
John Libbey Eurotest Ltd, 6 rue Blanche, 92120
Montrouge, France (1) 47 35 85 52
Excerpt. Pages 103-106.
Table of Contents
V
MAGNESIUM AND THERAPEUTICS
Magnesium has for a long time had only a modest place in
therapeutics, being used only for its cathartic or neutralizing
properties in oral preparations or for its sedative or
antispasmodic properties parenterally.
Today continually increasing knowledge of magnesium deficit is
allowing us to determine in what circumstances and in what manner
we can remedy it using physiologic doses of magnesium. Correct
use of magnesium therapy must prevent magnesium overload
accidents for which we must also still know the treatment. In a
few specific cases, parenteral and local magnesium therapy and,
even still more rarely, the induction of magnesium deficit show
why they should he used.
We will consider the following in order:
• The dietetic and medicinal
elements of magnesium therapy.
•The indications for and
techniques of oral magnesium therapy, less in strong cathartic
doses than in palliative physiologic doses for magnesium
deficit.
• The indications for and
techniques of parenteral and local magnesium therapy.
• The treatment of magnesium
overload.
• The exceptional case of
induction of a therapeutic magnesium deficit.
• Finally, the absolute and
relative contraindications for magnesium therapy.
1. The dietetic and medicinal
elements of magnesium therapy
In developed countries magnesium intake in the diet is
inadequate.
We will consider successively,
• the causes of this relative
deficiency,
• research on correcting it by
changes in diet.
• the particular value of
amounts in drinking water.
-
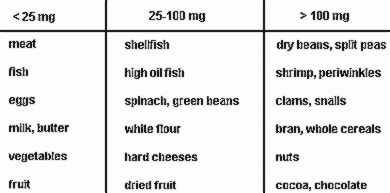
1.1 Dietetic
magnesium
-
-
1.2 Magnesium
salts
We will first describe the nature of the magnesium salts
that are available for use in therapy and then show how
difficult it is to compare them in their oral prescriptions
for remedying magnesium deficit. We will then review briefly
the pharmacodynamic properties of parenteral or local
magnesium.
-
-
1.21 The
characteristics of magnesium salts
In addition to simple magnesium oxide, a large number
of magnesium salts have been used therapeutically, either
mineral (chloride, sulfate, nitrate, carbonate) or
organic (acetate, bicitrate, methionate, levulinate,
ascorbate, glycero-phosphate, gluconate, aspartate,
propionate, lactate, fumarate, glutamate,
pyrrolidone-carboxylate).
Orally, soluble salts are thought to he better
tolerated and absorbed than insoluble salts (521, 522,
748, 1267) and organic salts more active than mineral
salts (691).
The use of dolomite as a source of natural magnesium
should be prohibited since this mineral can be a source
of toxic metals (Pub, Hg, Cd ...) (1086).
In reality, comparing aspects of the oral intake of
physiologic doses of different magnesium salts depends on
the conditions of the magnesium deficit for which they
are being compared. We can disregard the specific
properties of their anions since only the amount of
cation that is absorbed is significant. If it is a
question of correcting a deficiency, of which the most
typical form is intake deficiency, all magnesium salts
have a comparable bioavailability with only slight
differences (255, 1043, 1044, 1373). In human therapy,
there is no great problem in reducing such a deficiency
since it is sufficient to increase intake of the
deficient cation. But the difficult problem in magnesium
therapy consists in correcting deficits where there is,
at least partially, a depletion. One must then try to
rank magnesium salts, determining those that are the best
absorbed and penetrate the deficient tissues the best
since these are the most active and are retained the
best.
-
1.22
Bioavailability and therapeutic effectiveness of
physiologic doses of oral magnesium
Since magnesium salts are poorly absorbed, it would be
valuable to have magnesium salts of high
bioavailability.
The classic measure of the bioavailability of a drug
depends on the variations of its level in plasma after
oral and intravenous administration. This is not possible
for a physiologic parameter like plasma magnesium that is
subject to strict homeostatic control.
The use of isotopes does not resolve this problem
either. 28Mg has too short a half life and the
stable isotope 26Mg exists at a high
endogenous level which makes its use as a marker
difficult (1155, 1156, 1157, 1158).
One can however with a subject who is in neutral
magnesium balance and is on a diet with controlled ionic
intake consider an increase in urinary magnesium as a
positive indication of absorption, especially if it is
verified by initial hourly sampling which leaves little
time for a hypothetical tissue accumulation (534, 713,
778, 945).
In the absence of animal models of magnesium depletion
that are close to the cases found in humans, one can try
to compare the rate and amount of absorption and the
penetration and activity of different magnesium salts in
various tissues of animals made deficient in magnesium.
The quickest and most adequate correction of the
parameters of deficiency in animals — biologic (Mg
in plasma. erythrocyte. urine and the femur, Mg/Ca and
cAMP/cGMP ratios...), neurophysiologic (e.g., the study
of spontaneous motility) and cardiovascular... —
should allow us to rank different treatments even though
still inadequate, for magnesium depletion. Using a method
of this type to compare three magnesium salts for their
effects on initial and then cumulative urine levels of
magnesium during the first two days in the deficient rat,
it has been possible to show, as has been done in humans,
better absorption for MgCl2 than for
MgSO4 or magnesium oxide and better retention
for MgCl2, in relation to the same absorbed
amounts of the other two salts (938).
But this original experimental approach in animals
regarding the "therapeutic effectiveness" of a
physiologic dose of oral magnesium for magnesium
depletion can only be confirmed by the success of a
loading test. Intake of an oral physiologic dose (5
mg/kg/day) of a magnesium salt that leads to control of
subjective and objective parameters, tracings and dosages
for magnesium depletion constitutes the only proof of the
therapeutic effectiveness of a magnesium salt for
magnesium depletion. One can compare, to some extent at
least, a study of this type with one that shows the
superiority of pyrrolidone-carboxylate over sulfate in
volunteers that were theoretically healthy but had low
erythrocyte magnesium levels (894). However, given the
current state of our knowledge, no magnesium salt has
clearly shown its superiority in overt cases of magnesium
depletion.
-
1.23 The
pharmacodynamics of larges doses of magnesium given
orally or by parenteral or topical routes
While physiologic doses of magnesium do not have any
pharmacodynamic properties, magnesium in high oral doses
or given parenterally or topically does have
pharmacodynamic activity. The effects are particularly
well defined in iatrogenic magnesium overload during
massive parenteral magnesium therapy with its ability to
cause bradycardia and hypotension, its curariform and
ganglioplegic effects and its anticoagulant,
cytoprotective and calcium antagonist activity (204, 384,
756, 916, 933, 943). The comparative analysis of the
virtues of the different salts of magnesium in such
models is equally valuable for the corresponding
indications by these same routes and in these same doses
(83, 131, 204, 227, 541, 884). In contrast they do not
permit any extrapolation to the comparative virtues of
these same magnesium salts as therapeutic for magnesium
deficit in oral physiologic doses.
-
1.3 Magnesium-sparing
drugs
There are two types, those that cause low urinary
magnesium, thus reducing magnesium loss, and magnesium-fixing
agents of which the main activity is to enhance magnesium
penetration into the cell and its maintenance there.
-
-
1.31 Drugs that
reduce urinary magnesium
Two types of diuretics are capable of reducing urinary
magnesium, inhibitors of carbonic anhydrase and distal or
so called "potassium-sparing" diuretics.
-
-
1.311
Carbonic anhydrase inhibitors
Acetazolamide (332, 1281) and metazolamide (154,
332) combine reduction of urinary magnesium levels
with induction of metabolic acidosis (due to loss of
bicarbonate and retention of H+). It may
help in reducing neuromuscular hyper-excitability.
These carbonic anhydrase inhibitors often cause low
potassium levels. Administration of potassium salts
may reduce this.
-
1.312
"Potassium-sparing" distal tubule
diuretics
These can he divided into aldosterone antagonists
(spironolactone and canrenone) and distal diuretics
with direct renal action (triamterene and amiloride)
(363).
Among the aldosterone antagonists, spironolactone
is the most active in regard to magnesuria. Moreover
it exerts interesting general effects on ionic
transfers (883). But it also has many side
effects.
Among the direct distal diuretics, we prefer
amiloride, since triamterene creates the risk of
precipitation (779) and does not seem to maintain its
effects over the long term (1332).
-
1.313
Anti-stress drugs
In addition to drugs classified as specific renal
agents that lower urinary magnesium, we must consider
treatments for stress as true agents that lower
urinary magnesium indirectly. Stress is in fact one
of the major causes of renal loss of magnesium. The
frequency of its association with any type of
magnesium deficit demands that anti-stress treatments
which are logically used with therapy for the forms
of magnesium deficit that are secondary to this
dysfunction be mentioned among the customary agents
for lowering urinary magnesium.
Beyond their important specific effects that are
unrelated to magnesium, i.e., on neuromuscular
hyperexcitability, the usefulness of hygienic
prescriptions, psychotherapy, relaxation drugs,
muscle relaxants, anti-lipolytic agents and sedative
or autonomic nervous system treatments must be judged
by their effects in reducing urinary magnesium
levels.
-
1.32
Magnesium-fixing compounds
Among magnesium-fixing compounds the most often used
are vitamin B6 and vitamin D.
The intake of sulfur amino acids (cystine,
methionine), of glutathione and the vitamins needed in
their metabolism (B6, C) which tends to
increase available taurine is of only theoretical
interest.
The use of insulin and progesterone is limited by
their specific properties.
-
-
1.321
Vitamin B6
There are close links between B6 and
Mg. The formation of complexes between Mg,
B6 and amino acids permits active
transport of these three compounds and places the
vitamin at the head of the list of magnesium-fixing
agents. Moreover magnesium is necessary for the
activating phosphorylation of vitamin B6.
B6 and Mg are both necessary for protein
synthesis, especially for enzymes and are involved in
numerous physiologic functions at more or less
closely connected steps which allows their
association to have frequent simple or reinforcing
synergic effects: psycho-neurosedative, muscle
relaxing, cardiovascular and liver protective,
antihypoxic or antilithiasis, etc. (6, 97, 213, 333,
358, 363, 364, 499,726, 733, 746, 747, 844, 845, 967,
1374, 1375).
To obtain the best magnesium-fixing effects the
best ratio of vitamin B6 to physiologic
doses of Mg2+ is 2.5 parts pyridoxine
hydrochloride to one part magnesium ion. One has to
use pharmacologic doses, and not physiologic doses
(743), of vitamin B6 while remaining well
below those that have caused problems of excess,
i.e., more than two grams a day such as in
B6 megavitamin therapy (1141). This method
of magnesium therapy with its precise pharmacodynamic
doses of vitamin B6 has never caused such
problems in spite of its widespread use (101,155,
213, 332, 333, 345, 346, 358, 363, 364, 371, 380,
401a, 484, 726).
-
1.322 The
D vitamins
The physiologic forms of vitamin D (D3
or cholecalciferol) or the synthetic forms
D2 (or ergocalciferol) as well as their
hydroxylated derivatives whether physiologic
25-(OH)D3. (or calcidiol) and
1,25-(OH)2D3, the hormone-like
vitamin (or calcitriol) or synthetic,
1-(OH)D3 (or Unalpha®) promote the
passage of extracellular Mg into the tissues. As such
the D vitamins can be classified as magnesium fixing
agents. Moreover they increase the absorption of
magnesium (318, 332, 345, 358, 363, 364, 371, 380,
713, 1211). Unfortunately the D vitamins have as
their main effect increasing the absorption and
accumulation of calcium. Beyond the risks of these
properties that promote calcinosis, they increase
urinary magnesium either by raising blood calcium
levels or by a specific renal effect. Depending on
the degree of this urinary magnesium loss in relation
to their other effects on magnesium retention, the D
vitamins may or may not be used as magnesium-fixing
agents (318, 322, 332, 345, 358, 363, 364, 371,
380).
-
1.323
Sulfur compounds
Since taurine has magnesium-sparing properties
(361, 398, 401), it is tempting to try to increase
available levels of it. Because this sulfonic amino
acid is poorly absorbed, it is logical to increase
its precursors and to try to reduce their utilization
for synthesis of glutathione. Intake of methionine,
cystine and glutathione is therefore theoretically
desirable (397). Vitamins B6 and C (in
addition to their own therapeutic value) should favor
these metabolic goals (358, 397, 997).
-
1.324
Insulin
Insulin unquestionably facilitates entry into the
cell of the two principal intracellular cations, Mg
and K (1356) as well as that of the two major
alkaline earth elements, Mg and Ca (396). Since there
is much more free K+ (87%) than free Mg in
the cell (30%), insulin acts more on potassium
metabolism than on magnesium metabolism (336, 1356).
By adding a calcium ionophore effect to its magnesium
ionophore capacity, insulin combines with the desired
magnesium effect an action that is harmful in
relation to cellular calcium overloading during
magnesium deficit. Moreover it may cause tetany
through its own action on membranes even without
hypoglycemia (332). It is therefore a less than
perfect magnesium-fixing agent and its use is
necessarily limited to the parenteral1 (332,
336).
-
1.325
Progesterone
Experimentally progesterone causes magnesium
retention in the rat (519). If this fact is confirmed
in women in the particular cases where this hormone
is useful, such as, for example, premenstrual
syndrome and certain pregnancies that are at risk,
progesterone can be used as a magnesium-fixing
agent.
Its positive effect on magnesium balance does not
occur with progestagens like norethisterone
(519).
-
1.4 Partial magnesium
"analogues"
Perfect magnesium "analogues" should be able to relieve
all the effects of magnesium deficit without magnesium.
If no substance can today meet such a definition, partial
magnesium "analogues" are capable of replacing magnesium to
some extent in counteracting the effects of magnesium
deficiency.
Vitamin B6 would only deserve such a
classification if one thought pyridoxine could be equated
with vitamin G (333) since the latter may control tetany due
to magnesium deficit in the rat (526).
-
-
1 .41
Propranolol
Propranolol, by non-specific effects on membrane
stability (363, 394) and perhaps also by specific effects
that re-establish the activity of beta-adrenergic
dependent magnesium channels (204, 457) may occasionally
meet such a definition. This possibility corresponds to a
minority of the cases of latent tetany due to magnesium
deficit which propranolol controls (309).
-
1.42 Calcium
antagonists
Verapamil and nifepidine both partially control
various effects of experimental magnesium deficiency (28,
32, 208, 228, 457, 961). In symmetry with the idea that
attributes many of the effects of magnesium to its
physiologic action as a calcium antagonist (204, 756,
943), intake of calcium antagonists has been used to try
to counteract magnesium deficit as a factor in tissue
calcium overload. If there is currently no one that
relieves all the consequences of magnesium deficit, the
cardiac effects of the deficit seem to be the most
sensitive to this therapeutic remedy. Verapamil seems to
be the most active of calcium antagonists as a partial
magnesium "analogue" in spite of its different sites of
action (1006).
-
1 .43
Anticonvulsants
Various anticonvulsants of different chemical families
and different mechanisms of action (phenytoin, valproic
acid, baclofen, phenobarbital, clonazepam, carbamazepine)
are capable of controlling the major manifestations of
neuromuscular hyperexcitability due to magnesium deficit
(67, 363, 394). They can thus he classified as partial
magnesium "analogues" and magnesium deficiency can he
classified among the models for studying anticonvulsants
(18, 67, 175, 749, 1012). But they have no effect on the
other manifestations nor on the final prognostication of
the deficiency. We must however reserve a special place
for phenytoin due to its concomitant antitetanic and
anti-arrhythmic properties (363, 370, 394, 399, 723) and
for baclofen because of its muscle relaxing ability (158,
363, 1021, 1132).
The utilization of these partial magnesium "analogues"
that are particularly effective for certain aspects of
magnesium deficit has a role of choice among the adjuvant
treatments of the corresponding clinical forms that are
resistant to magnesium therapy.
This page was first uploaded to The Magnesium Web Site on July
13, 2002
http://www.mgwater.com/