Exhibit K
Dietary Reference Intakes
Calcium, Phosphorus, Magnesium, Vitamin D, and
Fluoride
National Academy of Science, Institute of Medicine,
1997
(Page 6-1)
VI
Magnesium
BACKGROUND INFORMATION
Overview
Total body magnesium (Mg) content
is approximately 25 g (1000 mmol), of which 50 to 60 percent
resides in bone, in the normal adult. One-third of skeletal
magnesium is exchangeable, and it is this fraction that may serve
as a reservoir for maintaining a normal extracellular magnesium
concentration (Elin, 1987). Extracellular magnesium accounts for
about 1 percent of total body magnesium. The normal serum
magnesium concentration is 0.75 to 0.95 mmol/liter (1.8 to 2.3
mg/dl).
Magnesium is a required cofactor
for over 300 enzyme systems (Wacker and Parisi, 1968). It is
required for both anaerobic and aerobic energy generation and for
glycolysis, either indirectly as a part of the Mg-ATP complex or
directly as an enzyme activator (Garfinkel and Garfinkel, 1985).
Magnesium has also been shown to be required for mitochondria to
carry out oxidative phosphorylation (Wacker and Parisi, 1968).
The mitochondrial enzymes utilize the magnesium chelate of ATP
and ADP as the actual substrates for phosphate transfer
reactions.
Magnesium transport into or out
of cells appears to require the presence of carrier- mediated
transport systems (Gunther, 1993; Romani et al., 1993). The
efflux of magnesium from the cell is coupled to sodium transport
and requires energy. Magnesium influx also appears to be linked
to sodium and bicarbonate transport but by a different mechanism.
The molecular characteristics of the magnesium transport proteins
have not been described.
Magnesium transport in mammalian
cells may be influenced by hormonal and pharmacological factors
including β-agonists, growth factors, and insulin (Gunther,
1993; Hwang et al., 1993; Romani et al., 1993). It has been
suggested that a hormonally regulated magnesium uptake system
controls intracellular magnesium concentration in cellular
compartments. The magnesium concentration in these compartments
would then serve to regulate the activity of magnesium-sensitive
enzymes.
(Page
6-2)
Magnesium presence is important
for maintaining an adequate supply of purine and pyrimidine
nucleotides required for the increased DNA and RNA synthesis that
occurs during cell proliferation (Rubin, 1975; Switzer, 1971).
Replicating cells must be able to synthesize new protein, and
this synthesis has been reported to be highly sensitive to
magnesium depletion. Many hormones, neurotransmitters, and other
cellular effectors regulate cellular activity via the adenylate
cyclase system, and the activation of adenylate cyclase requires
the presence of magnesium. There is also evidence for magnesium
binding through which magnesium directly increases adenylate
cyclase activity (Maguire, 1984).
Magnesium is necessary for
sodium, potassium-ATPase activity, which is responsible for
active transport of potassium (Dorup and Clausen, 1993).
Magnesium regulates the outward movement of potassium in
myocardial cells (Matsuda, 1991). The arrhythmogenic effect of
magnesium deficiency may be related to magnesium's role in
maintaining intracellular potassium.
Magnesium has been called
"nature's physiological calcium channel blocker" (Iseri and
French, 1984). During magnesium depletion, intracellular calcium
rises. Since calcium plays an important role in skeletal and
smooth muscle contraction, a state of magnesium depletion may
result in muscle cramps, hypertension, and coronary and cerebral
vasospasms. Magnesium depletion is found in a number of diseases
of cardiovascular and neuromuscular function, in malabsorption
syndromes, in diabetes mellitus, in renal wasting syndromes, and
in alcoholism (Ma et al., 1995). These observations have led to
studies regarding the role of inadequate magnesium intake in the
development of disease, as opposed to abnormal handling of
magnesium caused by the disease process. It is important to
ensure that such evaluations are undertaken in apparently normal
individuals for whom dietary intake is the primary independent
variable.
Physiology of Absorption, Metabolism, and
Excretion
In both children and adults,
fractional intestinal magnesium absorption is inversely
proportional to the amount of magnesium ingested (Kayne and Lee,
1993). In balance studies, under controlled dietary conditions in
healthy older men, an average of 3 8 0 mg (15.8 mmol)/day of
ingested magnesium resulted in net absorption of approximately 40
to 60 percent; true absorption ranged from 51 to 60 percent for
various foodstuffs when subjects were on a constant diet
(Schwartz et al, 1984). Net absorption has been estimated to be
15 to 36 percent at higher daily intakes (550 to 850 mg [22.9 to
35.4 mmol]) and with varying levels of dietary bran and oxalate
(Schwartz et al., 1986). Magnesium is absorbed along the entire
intestinal tract, but the sites of maximal magnesium absorption
appear to be the distal jejunum and ileum (Kayne and Lee, 1993).
Both an unsaturable passive and saturable active transport system
for magnesium absorption may account for the higher fractional
absorption at low dietary magnesium intakes (Fine et al., 1991
a).
A principal factor that regulates
intestinal magnesium transport has not been described. Vitamin D
and its metabolites 25-hydroxyvitamin D (25(OH)D) and
1,25-dihydroxyvitamin D (1,25(OH)2D) enhance intestinal magnesium
absorption to a small extent (Hardwick et al, 1991; Krejs et al.,
1983). Recently, a low magnesium diet in rats was shown to
increase 'J intestinal calbindin-D9k. Although these preliminary
data suggest a role for this vitamin D- (Page
6-3) dependent, calcium-binding protein in intestinal
magnesium absorption, the severe magnesium deficiency imposed may
have resulted in renal damage (not described) (Hemmingsen et al.,
1994).
The kidney is the principal organ
involved in magnesium homeostasis (Quamme and Dirks, 1986). The
renal handling of magnesium in humans is a
filtration-reabsorption process; there is no tubular secretion of
magnesium. Approximately 65 percent of filtered magnesium is
reabsorbed in the loop of Henle and 20 to 30 percent in the
proximal convoluted tubule (Quamme and Dirks, 1986). Magnesium
reabsorption in the proximal convoluted tubule appears to be
passive; it follows changes in salt and water reabsorption and is
associated with the rate of fluid flow. In the loop of Henle,
there appears to be an additional active transport system: a
decrease in magnesium reabsorption in this segment is independent
of sodium chloride transport in either hypermagnesemia or
hypercalcemia (Quamme, 1989). In vivo studies in animals and
humans, however, have demonstrated a tubular maximum for
magnesium that probably reflects a composite of these tubular
reabsorptive processes (Quamme and Dirks, 1986).
During experimental magnesium
depletion in humans, magnesium decreases in the urine to very low
levels (< 20 mg [1 mmol]/day) within 3 to 4 days (Fitzgerald
and Fourman, 1956; Heaton, 1969; Shils, 1969). Despite the close
regulation of magnesium by the kidney, no one has described a
hormone or factor that is responsible for renal magnesium
homeostasis. Because patients with either primary hyper- or
hypoparathyroidism usually have normal serum magnesium
concentrations and a normal tubular maximum for magnesium, it is
probable that parathyroid hormone (PTH) is not an important
regulator of magnesium homeostasis (Rude et al., 1980). Glucagon,
calcitonin, and ADH affect magnesium transport in the loop of
Henle in a manner similar to PTH, but the physiological relevance
of these actions is unknown (Quamme and Dirks, 1986). Little is
known about the effect of vitamin D on renal magnesium
handling.
Excessive alcohol intake has been
shown to cause renal magnesium wasting, which, if a diet is
marginal in magnesium content, could place an individual at risk
for magnesium depletion. Indeed, nearly all chronic alcoholics
have symptoms of magnesium depletion (Abbott et al., 1994).
However, the evidence does not substantiate the suggestion that
alcoholism is due to magnesium deficiency.
A growing list of medications has
been found to result in increased renal magnesium excretion.
Diuretics commonly used in the treatment of hypertension, heart
failure, and other edematous states may cause hypermagnesuria
(Ryan, 1987).
Factors Affecting the Magnesium
Requirement
Bioavailability
As mentioned previously, net
absorption of dietary magnesium in a typical diet is
approximately 50 percent. High levels of dietary fiber from
fruits, vegetables, and grains decrease magnesium absorption
and/or retention (Siener and Hesse, 1995; Wisker et al., 199 1).
Men consuming 355 mg (14.8 mmol)/day of magnesium were in
positive magnesium balance on a low-fiber (9 g/day) diet but in
negative balance on a high-fiber (59 g/day) diet (Kelsay et al.,
1979). Similar trends were observed in young women consuming 243
to 252 (Page
6-4) mg (10.0 to 10.5 mmol)/day of magnesium and receiving
a lower fiber (23 g/day) versus higher fiber (39 g/day) diet
(Wisker et al., 1991).
Nutrient-Nutrient Interactions
Phosphorus. Many foods
high in fiber contain phytate, which may decrease intestinal
magnesium absorption, probably by binding magnesium to phosphate
groups on phytic acid (Brink and Beynen, 1992; Franz, 1989;
Wisker et al., 1991). The ability of phosphate to bind magnesium
may explain decreases in intestinal magnesium absorption seen in
subjects on high phosphate diets (Franz, 1989; Hardwick et al.,
1991; Reinhold et al., 1991).
Calcium. Most human
studies of effects of dietary calcium on magnesium absorption
have shown no effect (Fine et al., 1991a; Hardwick et al., 1991;
Spencer et al., 1978b), but one has reported decreased magnesium
absorption rates (Greger et al., 1981). Perfusion of the jejunum
of normal subjects with 0 to 800 mg (0 to 20 mmol) calcium had no
effect on magnesium absorption (Brannan et al., 1976). Increased
calcium intake did not affect magnesium balance when as much as
2,000 mg (50 mmol)/day of calcium was given to adult men (Spencer
et al., 1978b, 1994), or when an additional 1,000 mg (25
mmol)/day of calcium was given to adolescents (Andon et al.,
1996). Magnesium intake ranging from 241 to 826 mg (10 to 34.4
mmol)/day did not alter calcium balance at either 241 mg (10
mmol) or 812 mg (20.3 mmol)/day of calcium (Spencer et al.,
1994). However, intakes of calcium in excess of 2,600 mg (65
mmol)/day have been reported to decrease magnesium balance
(Greger et al., 1981; Seelig, 1993). Several studies have found
that high sodium and calcium intake may result in increased renal
magnesium excretion (Kesteloot and Joossens, 1990; Martinez et
al., 1985; Quamme and Dirks, 1986), which may be secondary to the
interrelationship of the proximal tubular reabsorption of
filtered sodium, calcium, and magnesium (Quamme and Dirks, 1986).
Overall, at the dietary levels recommended in this report, the
interaction of magnesium with calcium is not of concern.
Protein. Dietary protein
may also influence intestinal magnesium absorption; magnesium
absorption is lower when protein intake is less than 30 g/day
(Hunt and Schofield, 1969). A higher protein intake (94 g/day)
may increase renal magnesium excretion (Mahalko et al., 1983),
presumably because an increased acid load increases urinary
magnesium excretion (Wong et al., 1986). However, the increased
urinary magnesium excretion did not change overall magnesium
retention, which indicates an ability of subjects to adapt to
this level of protein given the level of magnesium provided (258
mg [10.8 mmol]/day). Other studies in adolescents have shown
improved magnesium absorption and retention when protein intakes
were higher (93 versus 43 g protein/day) (Schwartz et al.,
1973).
Special Populations
Physical Activity.
Dietary magnesium intake in athletes has been reported to be at
or above recommended intakes (Clarkson and Haymes, 1995; Kleiner
et al., 1994; Niekamp and Baer, 1995), presumably due to their
higher food intake. Plasma/serum magnesium (Page
6-5) concentrations have been reported to fall with
chronic endurance exercise activity, while red blood cell values
appear to rise (Deuster and Singh, 1993). Although the decrease
in plasma magnesium has been suggested to reflect magnesium
depletion in athletes (Clarkson and Haymes, 1995), no clear
demonstration of magnesium depletion directly related to exercise
has been shown. Magnesium supplements did not enhance performance
in a study of marathon runners (Terblanche et al., 1992).
Intake of Magnesium
The U.S. Department of
Agriculture Continuing Survey of Food Intakes by Individuals
(CSFII) in 1994, adjusted by the method of Nusser et al. (1996),
indicated that the mean daily magnesium intake in males aged 9
and older was 323 mg(13.5 mmol) (fifth
percentile = 177 mg [7.4 mmol]; fiftieth percentile = 310 mg
[12.9 mmol]; ninety-fifth percentile = 516 mg [21.5 mmol])
(Cleveland et al., 1996) (see Appendix D for data tables). The
mean daily intake for females aged 9 and older was 228 mg [9.5 mmol) (fifth percentile = 134 mg [5.6
mmol]; fiftieth percentile = 222 mg [9.3 mmol]; ninety-fifth
percentile = 342 mg [14.3 mmol)). In both sexes, intake decreased
at age 70 and older. These intakes were similar to those found in
the National Health and Nutrition Examination Survey (NHANES) III
from 1988-1991 (Alaimo et al., 1994). National survey data for
Canada are not currently available. Other surveys have reported
lower intakes in both men and women (Hallfrisch and Muller,
1993).
The NHANES III study demonstrated
ethnic differences in intake. In that report, non- Hispanic black
subjects were found to consume less than either non-Hispanic
white or Hispanic subjects. Another study demonstrated that
elderly Hispanic males consumed a mean of 237 62 mg: (9.9
± 2.6 mmol)/day, while Hispanic females consumed a mean of
232 ± 71 mg (9.7 3.0 mmol)/day (Pluckebaum and Chavez,
1994).
Food and Water Sources of Magnesium
Magnesium is ubiquitous in foods,
but the magnesium content of foods varies substantially. Because
chlorophyll is the magnesium chelate of porphyrin, green leafy
vegetables are rich in magnesium. Foods such as unpolished grains
and nuts also have high magnesium content, whereas meats,
starches, and milk are more intermediate. Analyses from the 1989
Total Diet Study of the U.S. Food and Drug Administration
indicated that approximately 45 percent of dietary magnesium was
obtained from vegetables, fruits, grains, and nuts, whereas about
29 percent was obtained from milk, meat, and eggs (Pennington and
Young, 1991). Refined foods generally have the lowest magnesium
content. With the increased consumption of refined and/or
processed foods, dietary magnesium intake in the United States
appears to have decreased over the years (Marier, 1986). Total
magnesium intake is usually dependent on caloric intake, which
explains the higher intake levels seen in the young and in adult
males and the lower levels seen in women and in the elderly.
Water is a variable source of
intake; typically, water with increased "hardness" has a higher
concentration of magnesium salts. Since this varies depending on
the area from which water comes, much like fluoride, and the
manner in which it is stored, magnesium intake (Page
6-6) from water is usually not estimated except in
controlled diet studies. This omission may lead to
underestimating total intake and its variability.
Intake from Supplements
Based on a national survey in
1986, 14 percent of men and 17 percent of women in the United
States took supplements containing magnesium (Moss et al., 1989).
Approximately 8 percent of young children (2 to 6 years of age)
used magnesium-containing supplements. Women and men who use
magnesium supplements took similar doses, about 100 mg (4.2
mmol)/day, although the ninety-fifth percentile of intake was
somewhat higher for women, 400 mg (16.7 mmol)/day, than for men,
who were taking 350 mg (14.6 mmol)/day. Children who took
magnesium had a median daily intake of 23 mg (1 mmol) and a
ninety-fifth percentile daily supplemental intake of 117 mg (4.9
mmol).
Effects of Inadequate Magnesium Intake
Severe magnesium depletion leads
to specific biochemical abnormalities and clinical manifestations
that can be easily detected. Hypocalcemia, is a prominent
manifestation of magnesium deficiency in humans (Rude et al.,
1976). Magnesium deficiency must become moderate to severe before
symptomatic hypocalcemia develops. Even mild degrees of magnesium
depletion, however, may result in a significant fall in the serum
calcium concentration, as demonstrated in a 3-week study of
dietary-induced experimental human magnesium depletion (Fatemi et
al., 1991).
Magnesium is also important in
vitamin D metabolism and/or action. Patients with hypocalcemia
and magnesium deficiency are resistant to pharmacological doses
of vitamin D, 1 a hydroxyvitamin D, and 1,25(OH)2D (for a review,
see Fatemi et al. [1991]).
Neuromuscular hyperexcitability
is the initial problem cited in individuals who have or are
developing magnesium deficiency (Rude and Singer, 1980). Latent
tetany, as elicited by a positive Chvostek's and Trousseau's
sign, or spontaneous carpal-pedal spasm may be present. Frank,
generalized seizures may also occur. Although hypocalcemia may
contribute to the neurological signs, hypomagnesemia without
hypocalcemia may result in neuromuscular hyperexcitability.
There is emerging evidence that
habitually low intakes of magnesium and resulting abnormal
magnesium metabolism are associated with etiologic factors in
various metabolic diseases. In considering data from such
studies, it is important to separate the identification of
associations between the effect of the disease on magnesium
status from the effect of inadequate intake on magnesium status
and subsequent risk of disease. The specific disease states in
which magnesium status is implicated are discussed in the
following sections.
Cardiovascular
In normal subjects, experimental
magnesium depletion results in increased urinary thomboxane
concentration, angiotensin II-induced plasma aldosterone levels,
and blood pressure-indicating a potential effect of magnesium
deficiency on vascular function (Nadler (Page
6-7) et al., 1993; Rude et al., 1989). Magnesium depletion
is associated with cardiac complications, including
electrocardiographic changes, arrhythmias, and increased
sensitivity to cardiac glycosides (Rude, 1993). Atrial and
ventricular premature systoles, atrial fibrillation, and
ventricular tachycardia and fibrillation have been reported in
hypomagnesemic patients (Hollifield, 1987; Rude, 1993).
Significantly higher retention rates after magnesium load tests
have been reported in patients with ischemic heart disease
compared to normal controls (Rasmussen et al., 1988). This
suggests that a low magnesium concentration may also play a role
in cardiac ischemia. However, the extent to which the disease
modifies the indicators of magnesium deficiency rather than the
deficiency resulting in the disease manifestations varies with
the symptom and the individual studied.
The development of atheromatous
disease has been associated with magnesium in epidemiological
observational studies. Areas with increased water hardness (which
is due to high calcium and magnesium content) tend to have lower
cardiovascular death rates (Altura et al., 1990; Hammer and
Heyden, 1980; Leoni et al., 1985; Luoma et al., 1983; Neri and
Johansen, 1978; Neri et al., 1985; Rubenowitz et al., 1996).
Problems with evaluating epidemiological studies have been
identified (Comstock, 1979), and some studies have not found such
an association (Hammer and Heyden, 1980; Leoni et al., 1985).
However, as presented by Tucker (1996) and Beaton (1996), a
congruence of positive studies may suggest an association of
dietary intake and disease. Animals on low magnesium diets
develop arterial wall degeneration and calcification as well as
hypertriglyceridemia, hypercholesterolemia, and atherosclerosis
(Altura et al., 1990; Orimo and Ouchi, 1990). Controlled human
studies that support this relationship are lacking.
Magnesium depletion in patients
with cardiac diseases may be due to concomitant medications, such
as diuretics, as well as to dietary magnesium depletion. Although
cardiac arrhythmia may be associated with the primary cardiac
disorders, magnesium depletion may further predispose to cardiac
arrhythmias by decreasing intracellular potassium.
Accumulation of magnesium may
reduce the morbidity and mortality of patients in the period
following myocardial infarction. Two large, placebo-controlled,
randomized, double- blind studies of patients with myocardial
infarction have shown that intravenous magnesium therapy reduces
the incidence of therapy-requiring arrhythmias to approximately
one-half that seen in control patients (Antman, 1996; Seelig and
Elin, 1996). In one study of patients with acute myocardial
infarction, magnesium therapy given before thrombolytic therapy
decreased mortality by 24 percent (Woods and Fletcher, 1994).
Another large study of myocardial infarction did not find
favorable effects of magnesium that was administered after
thrombolytic therapy (ISIS4, 1995). Debate currently centers over
the time of administration of magnesium in terms of its favorable
effects (for review see Amman [1996]). Evidence does not support
the concept that the patients were magnesium deficient prior to
onset of the acute attack, only that magnesium therapy was
beneficial to outcome.
Blood Pressure
Epidemiologic evidence suggests
that magnesium may play an important role in regulating blood
pressure (Ascherio et al., 1992; Joffres et al., 1987; Ma et al.,
1995; McCarron, 1983; Witteman, et al., 1989). In these studies,
populations that have low dietary (Page
6-8) intake of magnesium have been reported to have an
increased incidence of hypertension. In one of the earlier
studies, dietary intake of magnesium in 44 normotensive subjects
was significantly greater than intake in 46 untreated
hypertensive subjects (McCarron, 1983). In the Honolulu heart
study, magnesium intake was the dietary variable that had the
strongest association with blood pressure (Joffres et al., 1987).
In another nutritional survey of 58,218 Caucasian women, those
who reported intakes of less than 200 mg (8.33 mmol)/day of
magnesium had a significantly higher risk of developing
hypertension than did women whose intakes were greater than 300
mg (12.5 mmol)/day. In a large prospective study of 30,681 men
without diagnosed hypertension, dietary magnesium intake was
inversely related to systolic and diastolic blood pressure and to
change in blood pressure during a 4-year follow- up period
(Ascherio et al., 1992). In this study, however, only dietary
fiber had an independent inverse association. Another study of
15,248 subjects found that dietary magnesium intake was inversely
associated with systolic and diastolic blood pressure (Ma et al.,
1995).
Intervention studies with
magnesium therapy for hypertensive patients have led to
conflicting results. Several studies have shown a positive
blood-pressure-lowering effect of magnesium supplements (Dyckner
and Wester, 1983; Geleijnse et al., 1994; Motoyamo et al., 1989;
Widman et al., 1993; Witteman. et al., 1994); others have not
(Cappuccio et al., 1985; Sacks et al., 1995; Wallach and Verch,
1986; Yamamoto et al., 1995; Zemel et al., 1990). Other dietary
factors may also play a role. A recent study demonstrated that a
diet of fruits and vegetables, which increased magnesium intakes
from an average of 176 mg (7.3 mmol)/day to 423 mg (17.6
mmol)/day, significantly lowered blood pressure in adults who
were not classified as hypertensive (systolic blood pressure <
140 mm Hg; diastolic blood pressure < 95 nun Hg) (Appel et
al., 1997). The addition of nonfat dairy products to the high
fruit and vegetable diet, which increased calcium intake as well,
resulted in further lowering of blood pressure. Potassium intake
was also greatly increased in both dietary regimens studied.
One study of hypertensive
patients revealed low serum magnesium concentrations (Albert et
al., 1958). No difference was detected in serum magnesium levels
in other studies, however (Gadallah et al., 1991; Tillman and
Semple, 1988). In patients with essential hypertension, free
magnesium levels in erythrocytes were inversely related to both
the systolic and diastolic blood pressure (Resnick et al., 1984).
It is unclear whether the decrease in serum magnesium
concentration was due to magnesium depletion or to
pathophysiological events that lead to hypertension.
The possible relationship between
hypertension and magnesium depletion is an important
consideration, as the two coexist in a high proportion of
individuals with diabetes and alcoholism (Resnick et al., 1991).
However, the role of long-term dietary intake of magnesium in the
prevalence of hypertension seen in the United States and Canada
has not been established.
(Page 6-9)
Skeletal Growth and Osteoporosis
Magnesium plays a major role in
bone and mineral homeostasis and can also directly affect bone
cell function as well as influence hydroxyapatite crystal
formation and growth (Cohen, 1988).
Magnesium deficiency may be a
risk factor for postmenopausal osteoporosis. Significant
reductions in the serum magnesium and bone mineral content (BMC),
but not red blood cell magnesium concentration or bone magnesium
content, have been described in women with postmenopausal
osteoporosis compared to age-matched controls (Reginster et al.,
1989). No correlations were found in a 4-year clinical trial of
magnesium intake and BMC in pre- and postmenopausal women
consuming about 250 mg (10.4 mmol)/day of magnesium (Freudenheim
et al. 1986), or in four of five skeletal sites measured in
postmenopausal women also consuming an average of 25 3 mg
± 11 mg (10. 5 ± 0.4 mmol)/day of
magnesium (Angus et al., 1988).
In contrast, BMC of the radius in
postmenopausal Japanese-American women was weakly positively
correlated with magnesium intake (Yano et al., 1985), while
elderly women who consumed less than 187 mg (7.8 mmol)/day had a
significantly lower bone mineral density (BMD) compared with
women whose average magnesium intake from diet was more than 187
mg (7.8 mmol)/day (Tucker et al., 1995).
Two studies are available on the
effect of magnesium supplementation on osteoporosis. In women
with documented osteoporosis, supplementation with 750 mg (31.3
mmol) of magnesium for the first 6 months followed by 250 mg
(10.4 mmol) supplementation from the seventh to twenty-fourth
month increased radial BMD after 12 months, but no further change
was seen in BMD by the end of the second year (Stendig-Lindberg
et al., 1993). Supplementation with 500 mg (20.8 mmol) of
magnesium and 600 mg (15 mmol) of calcium in postmenopausal women
who were receiving estrogen replacement therapy and daily
multivitamin and mineral tablets resulted in increased calcaneous
BMD in less than a year when compared with the postmenopausal
women who received sex steroid therapy alone (Abraham and Grewal,
1990). These observations suggest that dietary magnesium may be
related to osteoporosis and indicate a need for further
investigation of the role of magnesium in bone metabolism (Sojka
and Weaver, 1995).
Diabetes Mellitus
Magnesium depletion in a few
studies has been shown to result in insulin resistance as well as
impaired insulin secretion, and thereby may worsen control of
diabetes (for review, see Paolisso et al. [1990]). An
experimental magnesium depletion study was conducted to examine
the development of insulin resistance. Normal male subjects were
given a controlled diet for three weeks in a depletion metabolic
study in which magnesium intake was 12 mg (0.5 mmol)/day.
Intravenous glucose tolerance tests performed at the beginning
and end of the 21 -day depletion indicated a significant decrease
in insulin sensitivity (Nadler et al., 1993). Such findings have
raised the possibility that insulin resistance and abnormal
glucose tolerance in individuals may be due to inadequate
magnesium (Paolisso et al., 1992). Magnesium depletion in
clinical observational studies has been defined by low serum
magnesium concentrations as well as a reduction of total and/or
ionized magnesium in red (Page
6-10) blood cells, platelets, lymphocytes, and skeletal
muscle (Nadler et al., 1992), in spite of subjects consuming a
level of magnesium similar to that in population studies (Schmidt
et al., 1994).
Insulin resistance is commonly
noted in the elderly (Fink et al., 1983; Rowe et al., 1983).
Dietary magnesium supplements have been shown to improve glucose
tolerance (Paolisso et al., 1992) and improve insulin response in
elderly, non-insulin-dependent patients with diabetes (Paolisso
et al., 1989). One possible cause for the magnesium depletion
seen in diabetes is glycosuria-induced renal magnesium wasting
(Rude, 1993). However, until magnesium depletion studies
conducted in normal individuals can relate specific dietary
intake levels with abnormal glucose tolerance testing or other
indicators of glucose metabolism, it is premature to consider the
prevalence of diabetes mellitus as a functional indicator of
adequacy for magnesium.
Increased Risk in the Elderly
Several studies have demonstrated
that elderly people have relatively low dietary intakes of
magnesium (Goren et al., 1993; Lowik et al., 1993). Their lower
intake may be due to a variety of reasons, including poor
appetite, loss of taste and smell, poorly fitting dentures, and
difficulty in shopping for and preparing meals (Mountokalakis,
1987). Meals in some long-term care facilities may provide less
than the recommended levels of magnesium (Lipski et al., 1993).
In addition, magnesium metabolism may change with aging. As
mentioned earlier, intestinal magnesium absorption tends to
decrease with aging, and urinary magnesium excretion increases
(Lowik et al., 1993; Martin, 1990). A suboptimal diet in
magnesium may therefore place this population at risk for
magnesium depletion.
ESTIMATING REQUIREMENTS FOR MAGNESIUM
Selection of Indicators for Estimating the Magnesium
Requirement
Serum Magnesium
The serum magnesium concentration
may not reflect intracellular magnesium availability.
Nevertheless, measurement of the serum magnesium concentration is
the most available and commonly employed test to assess magnesium
status. The serum magnesium level may be influenced by changes in
serum albumin, other anionic ligands, and pH; however, correction
for changes due to these factors is seldom made (Quamme, 1993).
Normal values by age and gender have been derived from sampling
the U.S. population in NHANES I (Lowenstein and Stanton, 1986). A
serum magnesium concentration of less than 0.75 mmol/liter (1.8
mg/dl) is thought to indicate magnesium depletion (Elin,
1987).
Experimentally-induced dietary
magnesium depletion consistently leads to decreased serum
magnesium values in otherwise healthy humans. This suggests that,
under these circumstances, serum magnesium is a sensitive
indicator of magnesium status (Fatemi et al., 1991).
Hypomagnesemia (serum magnesium < 0.75 mmol/liter [1.8 mg/dl])
usually develops concurrently with moderate to severe magnesium
depletion. However, in clinical studies in (Page
6-11) which concentrations of magnesium in blood cells,
bone cells, or muscle cells are abnormally low (such as in
diabetes mellitus, alcoholism, or malabsorption syndromes), serum
magnesium values have been reported to be within the normal range
(Abbott et al., 1994; Nadler et al., 1992; Rude and Olerich,
1996). These findings suggests that intracellular magnesium is a
better guide to magnesium status in humans than is the
concentration in serum (Reinhart, 1988; Ryzen, et al. 1986). One
recent longitudinal evaluation of 8,251 subjects who entered a
study between 1971 and 1975 found 492 cardiovascular events when
the subjects were reevaluated between 1982 and 1984 (Gartside and
Glueck, 1995). Subjects with serum magnesium levels less than
0.81 mmol/liter (1.9 mg/dl) had greater risk of cardiovascular
disease than did those with a serum magnesium level greater than
0.87 mmol/liter (2.1 mg/dl). Although both values were within
what has been considered the normal range, 0.75 to 0.95
mmol/liter (1.8 to 2.3 mg/dl), (Elin, 1987), this variation in
response questions the validity of serum magnesium levels as
indicators of magnesium status (Gartside and Glueck, 1995). There
are also reports that elderly subjects may have a decrease in
magnesium status as determined by magnesium tolerance testing
(see below) despite normal serum magnesium concentrations. Thus,
serum magnesium concentration has not been validated as a
reliable indicator of body magnesium status.
Plasma-Ionized Magnesium
Recently, ion-specific electrodes
have become available for determining ionized magnesium in the
plasma. Early results suggest that this may be a better index of
magnesium status than the total serum magnesium concentration;
however, further evaluation is necessary. Few studies have been
conducted under varying conditions to assess its validity (Altura
et al., 1992; Mimouni, 1996).
Intracellular Magnesium
The total magnesium contents of
several tissues, including red blood cells, skeletal muscle,
bone, and peripheral lymphocytes have been evaluated as indices
of magnesium status. However, determination of intracellular
magnesium concentration should be a more physiologically relevant
measurement of magnesium status, as it is thought to play a
critical role in enzyme activation within the cell. In general,
poor correlation exists between the serum magnesium concentration
and intracellular levels (Salem et al., 1991; Whang et al.,
1994). The magnesium content of peripheral lymphocytes correlates
with skeletal and cardiac muscle magnesium content (Elin and
Hosseini, 1985), and seems to be a more accurate indicator of
magnesium status than the serum magnesium concentration (Ryzen et
al., 1986). However, lymphocyte magnesium content is probably not
a sufficiently discriminatory test to diagnose magnesium status
in any given individual.
Red blood cell magnesium has been
determined by nuclear magnetic resonance (Rude et al., 1991). The
fluorescence probe has been utilized for determination of free
magnesium in lymphocytes and platelets (Hua et al., 1995; Nadler
et al., 1992). Red blood cell magnesium values fall within days
following institution of a low-magnesium diet in normal subjects
(Fatemi et al., 1991). The mean intracellular free magnesium was
lower than normal in (Page
6-12) patients at high risk for magnesium depletion (for
example, individuals with diabetes or alcoholism) (Hua et al.,
1995; Nadler et al., 1992; Rude, 1991-1992).
In one study, total red blood
cell magnesium concentration was found to be lower in elderly
subjects (77.8 ± 2.1 years), as compared with younger
people (36.1 ± 0.4 years), when the mean magnesium
consumption of both groups was 311 ± 21 mg (13.0 ±
0.9 mmol)/day (Paolisso et al., 1992). However, the evidence was
not judged sufficient to use red blood cell magnesium as an
indicator of status.
Magnesium Balance Studies
The principal measure of adequate
dietary magnesium in the past has been the dietary balance study
(Greger and Baier, 1983; Hunt and Schofield, 1969; Mahalko et
al., 1983; Schwartz et al., 1984, 1986). Most balance studies
were performed at clinical research centers where the diet was
constant and controlled. However, this technique still presents
several problems, including the measurement of magnesium intake
and urine and stool magnesium excretion. In addition, sweat and
dermal losses of magnesium have not usually been considered.
In adults, balance studies should
be performed at magnesium intakes just below and above those at
which zero balance is achieved to obtain the approximately linear
dependence of loss and retention near balance (Beaton, 1996). The
intake associated with zero balance from each individual studied
can then be grouped and the variability of requirements estimated
(Beaton, 1996). Since magnesium intake is related to energy
intake (Clarkson and Haymes, 1995; Niekamp and Baer, 1995),
balance studies may report the findings in relation to estimated
energy requirements if the data are to be applied to the general
population. Magnesium may be obtained from food, water, nutrient
supplements, or pharmalogical agents, but the bioavailability may
differ.
Although numerous magnesium
balance studies have been performed, not all met the requirements
of a well-designed study. Some provided for a period of
adaptation but did not include magnesium intakes, which would
have allowed average requirements to be estimated. Balance
studies performed prior to 1960 utilized less accurate means to
measure magnesium as compared with atomic absorption
spectrophotometry. The minimum criteria used here for inclusion
of balance studies for the development of recommendations for
magnesium requirements included either an adaptation period of at
least 12 days or a determination of balance while the subjects
consumed self-selected diets. The disadvantage of using
self-selected diets is that only one level is being evaluated. If
the individual is in balance or nearly so, it is not possible to
discern if this is just an adaptation to that level or if it
truly represents the minimal level of adequacy. Similarly, if
only one level of intake is provided, no matter how accurate, it
is not possible get the dose response data necessary to estimate
the requirement. At least two levels need to be evaluated: one
below and one near the required level.
When a study is not carried out
in a metabolic unit or under close supervision, data are
generally lacking on magnesium intake from water. This omission
precludes the use of many of the earlier studies conducted in
free-living environments or current studies in which intakes were
calculated rather than analyzed.
(Page 6-13)
Estimates of Tissue Accretion during Growth
In the absence of data regarding
specific requirements for magnesium for growth during various
life stages, accretion rates of magnesium have been estimated
(Nordin, 1976; Widdowson and Dickerson, 1964). The rates of
tissue accretion during childhood are derived from analysis of
cadavers, and the utility of these data is limited. In some cases
using data from cadavers, the estimates of whole body mineral
retention must be calculated based on measurements from regional
sites (Fornon and Nelson, 1993). Fornon and Nelson (1993) and Koo
and Tsang (in press) used data from cadavers to estimate a net
accretion of 10 mg (0.4 mmol)/day during the second year of life.
However, further information is needed before this approach may
be uniformly used to estimate magnesium needs throughout
childhood.
The total magnesium content of an
infant weighing 3.5 kg (7.7 lb) is approximately 220 mg (9.2
mmol)/kg or 760 mg (32 mmol). Magnesium as a percentage of
fat-free body mass increases during gestation, but at birth the
percentage is much less than that of an adult (Widdowson and
Dickerson, 1964). The content of magnesium in an adult man is
estimated to total 27.4 g (1141.7 mmol), or about 390 mg (16.2
mmol)/kg (Widdowson and Dickerson, 1964).
In order to accumulate
approximately 26.6 g (1108.3 mmol) over the 20 years of growth
from infancy to adulthood, an average daily accretion during this
period of about 3.6 mg (0.2 mmol) would be necessary. However,
the growth rate is not linear with age. It has been suggested
that an adequate accretion rate (positive balance) for girls 10
to 12 years of age and weighing about 40 kg (88 lb) is 8.5 mg
(0.3 mmol)/day (Andon et al., 1996). For older children who are
heavier and experiencing greater growth in lean and bony tissue,
a positive balance in the range of 10 mg (0.4 mmol)/day would be
appropriate.
Magnesium Tolerance Test
The magnesium tolerance test,
which is based on the renal excretion of a parenterally
administered magnesium load, has been used for many years. It is
considered by some to be an accurate means of assessing magnesium
status in adults, but not in infants and children (Gullestad et
al., 1992; Ryzen et al., 1985). However, the sensitivity of this
method in detecting magnesium depletion may be different between
subjects with and without hypomagnesemia. In 15 hypomagnesemic
subjects, 85 ± 3 percent of a parenterally administered
magnesium load was retained, compared to only 14 ± 4
percent in 23 normal controls (Ryzen et al., 1985). In a group of
24 chronic alcoholics at risk of magnesium deficiency, retention
of 51 + 5 percent was also
significantly greater than the control group. While the magnesium
tolerance test has been shown in this and other studies (Cohen
and Laor, 1990; Costello et al., 1997; Gullestad et al., 1992) to
detect magnesium depletion in both hypomagnesemic and
normomagnesemic subjects at risk of magnesium depletion, the test
was not sensitive to detect treatment effects of magnesium
supplementation in otherwise healthy subjects (Costello et al.,
1997). After 3 months of supplementation of 350 mg (14.5
mmol)/day of magnesium, the mean retention of 37 percent did not
change significantly. Thus, the sensitivity of this method in
normal subjects is not yet validated and so cannot be accepted as
the primary indicator for assessing adequacy at this time.
(Page 6-14)
One of the problems in using the magnesium tolerance test is
that it requires normal renal handling of magnesium. Urinary
magnesium loss (related to conditions such as diabetes or drug or
alcohol use) may yield an inappropriate negative test. Moreover,
impaired renal function may result in a false positive test
(Martin, 1990). Age may also be a confounding variable, since
older subjects (73 ± 6 years) have been reported to retain
significantly more magnesium than younger subjects (33 ±
10 years), despite a comparable mean daily dietary magnesium
intake of 5.1 mg (0.2 mmol)/kg of body weight (Gullestad et al.,
1994). Supplements of 225 mg (9.4 mmol)/day of magnesium as
magnesium lactate-citrate for 30 days to the elderly subjects
turned the test results toward normal. Martin (1990) studied 30
elderly females (mean age of 82 years, range 72 to 93 years) who
were stated to have "lower than recommended dietary magnesium
intakes". Subjects with serum magnesium less than 0.59 ±
.07 mmol/liter (1.4 ± 0.2 mg/dl) retained a higher
percentage of the magnesium load (61 ± 12 percent) than
subjects with mean serum magnesium levels of 0.72 ± .02
mmol/liter (1.7 ± 0.05 mg/dl) whose retention was 43
± 16 percent. Both of these levels of retention, however,
are high compared to that seen in younger age groups. Thus, the
influence of renal function in this test cannot be ignored. Of
significant concern, even if this test is validated in future
studies as a primary indicator of magnesium status, is the
invasive procedure (intravenous administration) used.
Epidemiological Studies and Meta-analysis
As discussed in the previous
section, Epidemiologic studies have suggested that individuals or
groups ingesting hard water that contains magnesium, consuming a
diet higher in magnesium, or using magnesium supplements, have
decreased morbidity from cardiovascular disease or less
hypertension (Altura et al., 1990; Ascherio et al., 1992; Hammer
and Heyden, 1980; Joffres et al., 1987; Leoni et al., 1985; Luoma
et al., 1983; Ma et al., 1995; McCarron, 1983; Nadler et al.,
1993; Neri and Johansen, 1978; Neri et al., 1985; Rubenowitz et
al., 1996; Witteman et al., 1989). Low magnesium intake has also
been linked to osteoporosis (Sojka and Weaver, 1995). Because of
the difficulty of conclusively establishing that the lack of
dietary magnesium was the primary causative factor in these
chronic diseases, the basis for a claim is equivocal. Thus, based
on the scientific literature currently available, these data can
not serve as the indicators of adequacy in estimating magnesium
requirements. However, as recently reviewed by Tucker (1996), the
evidence from many studies, taken together, may lend confidence
to the theory that dietary magnesium intake may indeed contribute
to these disorders.
FINDINGS BY LIFE-STAGE AND GENDER GROUP
Birth to 12 Months
No functional criteria of magnesium status have been
demonstrated that reflect response to dietary intake in infants.
Thus, recommended intakes of magnesium are based on an Adequate
Intake (Al) that reflects the observed mean intake of infants fed
principally with human milk.
(Page
6-15)
Indicators Used to Set the AI
Human Milk. Human milk
is recognized as the optimal milk source for infants throughout
at least the first year of life and recommended as the sole
nutritional milk source for infants during the first 4 to 6
months of life (IOM, 1991). Therefore, determination of the Al
for magnesium for infants is based on data from infants fed human
milk as the principal fluid during periods 0 to 6 and 6 to 12
months of age. The Al is set at the mean value for observed
intakes as determined from studies in which intake of human milk
was measured by test weighing volume, and intake of food was
determined by dietary records for 3 days or more.
Balance Studies. The
limited data on magnesium balance in infants were considered
supportive evidence for the derived AL Many of the magnesium
balance studies involving human milk-fed infants have been
performed on premature infants or infants in the first weeks of
life. Net retention of magnesium in the studies of premature
infants was 10 to 15 mg (0.4 to 0.6 mmol)/day with 45 percent
absorption (Atkinson et al., 1987; Schanler et al., 19 8 5). This
level of retention was similar to that reported earlier in 10
breast-fed infants at 5 to 7 days of age, with repeat balance
studies performed in three of these infants again at 4 to 6 weeks
of age (Widdowson, 1965).
Differences in magnesium needs
between infants fed human milk and those fed infant formula are
described in the "Special Considerations" section.
Ages 0 to 6 Months
Based on data from a summary of
recent studies in North America and the United Kingdom (Atkinson
et al., 1995), the concentration of magnesium in human milk is
usually about 34 mg (1.4 mmol)/liter, and the concentration
remains relatively constant over the first year of lactation
(Allen et al., 1991; Dewey et al., 1984). The AI is set based on
a reported average intake of human milk of 780 ml/day (Allen et
al., 1991; Butte et al., 1984; Heinig et al., 1993) and the
average milk magnesium concentration of 34 mg (1.4 mmol)/liter.
This gives an Al of 30 mg (1.3 mmol)/day. The balance data cited
above support an Al of 30 mg (1.3 mmol)/day for this age range,
as it would allow infants to maintain a positive magnesium
balance of at least 10 mg (0.4 mmol)/day during early
infancy.
Ages 6 to 12 Months
During the second 6 months of
life, solid foods become a more important part of the infant diet
and add a significant but poorly defined amount of magnesium. The
absorption of magnesium from solid foods and the effects of solid
foods on absorption of magnesium from human milk are unknown. To
set an AI for infants from 6 to 12 months of age, the average
magnesium intake from solid foods-55 mg (2.2 mmol)/day-for 9- to
12-month-old formula-fed infants (Specker et al., 1997) was used.
This approach assumes that infants who are fed human milk have
intakes of solid food similar to those fed formulas.
(Page
6-16
Based on the data of Heinig et
al. (1993), the mean volume of human milk consumed between 7 and
11 months of age would be 600 ml/day. Thus, magnesium intake from
human milk with an average magnesium concentration of 34 mg (1.4
mmol)/liter would be about 20 mg (0.8 mmol)/day. Summing the
intake from human milk and from solid food, the Al for magnesium
for infants 6 to 12 months of age is set at 75 mg (3.1
mmol)/day.
| AI for
Infants |
0 to 6
month |
30 mg
(1.1 mmol)/day |
| |
6 to
12 months |
75 mg
(3.1 mmol)/day |
Special Considerations
Infant Formulas.
Commercial infant formulas that are cow milk-based are generally
higher in magnesium concentration, 40 to 50 mg (1.7 to 2.1
mmol)/liter, than human milk. Soy-based formulas may have even
higher concentrations of magnesium, 50 to 80 mg (2.1 to 3.3
mmol)/liter (Fornon and Nelson, 1993; Greer, 1989). In a large
series of studies (>300 balances), Fornon and Nelson (1993)
reported approximately 40 percent net absorption of magnesium in
infants fed soy or cow milk-based formulas with a net retention
of 10 mg (0.4 mmol)/day based on total intakes of 53 to 59 mg
(2.2 to 2.5 mmol)/day of magnesium.
Higher absorption values of 57 to
71 percent of magnesium intake from standard cow milk-based
infant formulas have been reported (Kobayashi et al., 1975; Moya
et al., 1992). A dietary fractional absorption rate of 64
± 4 percent was reported in three infants aged 4 to 10
months. Magnesium intakes of the infants exceeded 150 mg (6.3
mmol)/day in the latter study.
Direct assessment of an Al for
magnesium for formula-fed infants is not possible due to the lack
of data comparing magnesium absorption from human milk and from
infant formulas. Based on the current U.S. practices of infant
formula manufacturers, the increase of 20 percent above the level
of magnesium intake of human milk-fed infants allows for the
possibility of a lower bioavailability of magnesium from
formulas. This leads to an estimated intake of 35 mg (1.5
mmol)/day (human milk + 20 percent) for formula-fed infants.
Similar absorption of magnesium from soy versus routine formulas
(Fornon and Nelson, 1993) does not indicate a greater need for
magnesium in soy formula-fed infants, but the practice of
increasing magnesium intake in soy formulas relative to cow-milk
formulas is widely followed by formula manufacturers.
Ages 1 through 3, 4 through 8, 9 through 13, and 14
through 18 Years
Indicators Used to Set the
EAR
A possible approach to
determining human magnesium requirements is to estimate the
intake required to achieve a level of retention associated with a
beneficial outcome. Although magnesium retention during growth
should be positive, the desirable extent of retention for
magnesium is unknown. As discussed earlier, there are inadequate
data upon which to attribute a specific benefit to maximal
retention of magnesium. This is unlike calcium, for which maximal
retention can be associated with benefit to bone mass accretion.
(Page
6-17) Since the criterion of maximal retention could not
be used for magnesium, magnesium balance data were used as the
basis for establishing EARs for these age groups.
As mentioned previously, an
adequate accretion rate (positive balance) for girls 10 to 12
years of age and weighing about 40 kg (88 lb) may be 8.5 mg (0.3
mmol)/day (Andon et al., 1996). It is probable that for older
children, who are heavier and experiencing greater growth in lean
and bony tissue, a positive balance in the range of 10 mg (0.4
mmol)/day of magnesium would be appropriate. This would allow for
the greater need for magnesium during the specific periods of
faster growth during older childhood. In the absence of more
definitive goals, a daily positive balance of 8 to 10 mg (0.3 to
0.4 mmol) of magnesium seems to be a reasonable goal upon which
to base an EAR for growing children and adolescents.
Balance Studies. For
children under 10 years of age, there is only one report of a
balance study published since 1960 (Schofield and Morrell, 1960).
For children between 10 and 15 years of age, seven studies were
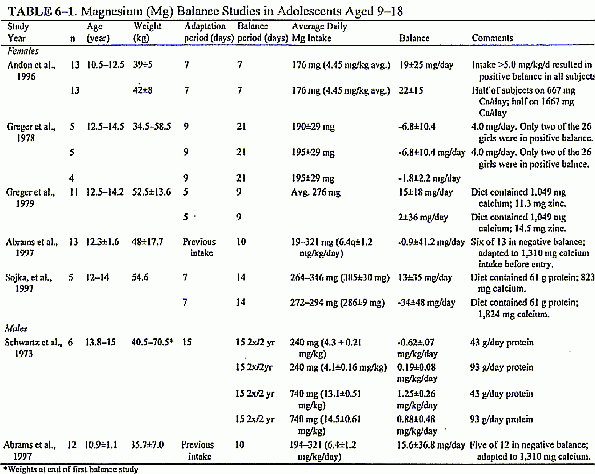
available for consideration (see Table 6-1). The amount of
magnesium lost via other routes (dermal, sweat, menses, and other
losses) was not measured or estimated in the calculations of any
of these studies. Other balance studies performed in children
prior to 1960 (see review by Seelig [1981]) were not considered
because information regarding absorption over a range of intakes
was not provided and results reported may not be reliable using
the analytical methodology available at that time.
Given the information provided in
the available balance studies, expression of magnesium
requirements for children is probably more accurate on the basis
of intake per day, rather than per unit of body weight or per
amount of lean tissue. When expressed on a mg/kg/day basis,
magnesium requirements determined by balance studies in subjects
who were obese were much lower than those in subjects of normal
weight (Jones et al., 1967), as fat contains less magnesium than
other tissues and body fat increases with age. Expressing
Estimated Average Requirements (EARs) and Recommended Dietary
Allowances (RDAs) per kg ideal body weight or lean body mass
would be more accurate than per kg total weight. However, since
most reports of balance studies do not provide individual intake
and body weight or height data, it is seldom possible to
determine the response of individuals to specific levels of
magnesium consumed on either an ideal or actual body weight
basis. Future experiments might address requirements in relation
to energy needs (Shils and Rude, 1996).
Because of the lack of studies in
younger children, the data from children 10 to 15 years old (see
Table 6-1) were extrapolated using reference body weights.
Interpretation of the available balance data are confounded by
the lack of information provided on individual body weights,
varying age and weight ranges studied within and between studies,
and variations in dietary protein (both in amount and source) and
calcium (see "Calcium" and "Protein" below). In reviewing the
details of the studies summarized in Table 6-1, it appears that,
provided the diet has adequate protein for 9 through 13 year
olds, group mean positive magnesium balance in the range of 10 mg
(0.41 mmol)/day is achieved at intakes of approximately 5 mg
(0.21 mmol)/kg/day. In the recent short-term study using
multitracer stable isotope technique to assess magnesium balance
in 13 adolescent girls (Abrams et al., in press), the mean
magnesium balance was slightly negative (-0.9 ± 41 mg
[0.04 ± 1.7 (Page
6-18) mmol]/day) at comparatively high levels of average
intake (6.4 mg [0.27 mmol]/kg/day of magnesium). Some of this may
have been due to the amount of calcium in the diet (1,3 10 mg [33
mmol]/day); however, another recent study using multitracer
stable isotopes (Sojka et al., in press), did not show a
significant difference in magnesium balance with two levels of
dietary calcium (see "Calcium" below).
In the one balance study in which
children 7 to 9 years old were evaluated, positive magnesium
balance was achieved on daily dietary magnesium intakes that
ranged from 121 to 232 mg (5.0 to 9.7 mmol)/day (Schofield and
Morrell, 1960); a magnesium intake of 5 mg (0.21 mmol)/kg/day
appeared to meet some but not all of the younger children's
needs. Taken together with the data on older children, the
available balance studies suggest that at a magnesium intake of 5
mg/kg body weight/day, some but not all children would be in
magnesium balance. The extent to which this represents 50 percent
of children in these age groups and pubertal stages meeting their
needs is difficult to predict because of the confounding
variables of other dietary components. For establishing the EARs
for magnesium for children aged 1 through 3 and 4 through 8, for
which balance studies are unavailable, the value of 5 mg/kg/day
is adopted.
Additional Factors Considered for the Above
Studies
Calcium. As stated
earlier, increasing calcium intake may have a negative effect on
magnesium balance (Greger et al., 1981). In two recent studies in
this age group, however, little effect was noted. Compared with a
calcium intake of 667 mg (16.7 mmol)/day, intakes of 1,667 mg
(41.7 mmol)/day (the additional 1,000 mg [25 mmol] from two
divided doses of a supplement) did not decrease magnesium
retention in girls aged 10 to 12 years (Andon et al., 1996). In a
magnesium kinetic study in which five girls aged 12 to 14 years
were given two stable isotopes of magnesium at two levels of
calcium, 800 mg (20 mmol) and 1,800 mg (45 mmol), no significant
differences were seen in the magnesium balances measured or in
the percentage of magnesium absorbed (Sojka et al., in press).
The calcium contents of the diets provided in the other two
balance studies of adolescents were 1,200 mg (30 mmol) (which
included supplements) (Schwartz et al., 1973) and 1,060 to 1,080
mg (26.5 to 27 mmol) from foods (Greger et al., 1978).
Supplementation with three sources of 900 mg (22.5 mmol) of
calcium to a controlled, food-based diet containing 300 to 350 mg
(12.5 to 14.6 mmol) and 697 mg (17.4 mmol) calcium/day did not
effect overall magnesium retention in eight adult male subjects
(Lewis et al., 1989).
Protein. Magnesium
requirements are influenced by the level of dietary protein,
apparently in part related to effects on urinary magnesium
excretion (Lakshmanan et al., 1984; Mahalko et al., 1983; Wisker
et al., 1991). Magnesium intakes in male adolescents on lower
protein intakes (50 g/day) were inadequate, but with higher
protein diets (94 g/day), balances became positive (Schwartz, et
al., 1973). Some have suggested that the effect of protein may
differ for animal and vegetable sources. However, in one study of
adolescent girls, little difference was noted in this age group
when soy was substituted for animal protein at a level of 30
percent of the total protein that was fed at a level of 46 to 49
g/day.
(Page 6-19)
(Page 6-20) (Greger et al., 1978). The influence
of protein intake on magnesium requirements needs additional
study.
EAR Summary: Ages 1 through 3 and 4 through 8
Years
In the absence of adequate
balance or usual accretion data in children aged 1 through 8
years, it is necessary to interpolate data from other age groups
based on changes in body weight and linear growth. Based on
studies in adolescents (Abrams et al., in press; Andon et al.,
1996; Greger et al., 1978, 1979; Schwartz et al., 1973) and the
one study in 7- to 9- year- old children (Schofield and Morrell,
1960), a magnesium intake of 5 mg (0.21 mmol)/kg/day appears to
have met some but not all the needs of those evaluated. This is
the basis for the EAR for children aged 1 through 3 years and 4
through 8 years. For children aged 1 through 3 years with a
reference weight of 13 kg (Table 1-3), the EAR is 65 mg (2.7
mmol)/day. For children aged 4 through 8 years with a reference
weight of 22 kg, the EAR is 110 mg (4.6 mmol)/day. It is
recognized that further studies specific to this age group are
needed before more precise EARs can be assigned or distinctions
made between males and females or between children of different
racial or ethnic groups.
| EAR
for Children |
1
through 3 years |
65 mg
(2.7 mmol)/day |
| |
4
through 8 years |
110 mg
(4.6 mmol)/day |
Based on the 1994 CSFII magnesium
intake data, adjusted for day-to-day variation (Nusser et al.,
1996), the first percentile of intake for children aged 1 through
3 years is 80 mg (3.3 mmol) (see Appendix D), which is above the
EAR of 65 mg (2.7 mmol)/day. The median intake for magnesium for
this age group is 180 mg (7.5 mmol). For children aged 4 through
8 years, the first percentile of intake is 103 mg (4.3 mmol)/day,
slightly below the EAR of 110 mg (4.6 mmol)/day. The median
magnesium intake is 206 mg (8.6 mmol)/day.
Determination of the RDA: Ages 1 through 3 and 4 through 8
Years
The variance in requirements
cannot be determined from the available data. Thus, a coefficient
of variation (CV) of 10 percent is assumed, which results in an
RDA for magnesium of 80 mg (3.3 mmol)/day for children aged 1
through 3 years, and an RDA of 130 mg (5.4 mmol)/day for children
aged 4 through 8 years.
| RDA
for Children |
1
through 3 years |
80 mg
(3.3 mmol)/day |
| |
4
through 8 years |
130 mg
(5.4 mmol)/day |
EAR Summary: Ages 9 through 13 Years, Boys
The Abrams et al. (in press)
study provides some data for boys ages 9 through 13 years that
leads to the conclusion that boys, not yet into maximal growth at
this age, appear to require about the same amount of magnesium as
girls per kg per day. Thus, the EAR is estimated to be 5 mg (0.21
mmol)/kg/day for boys aged 9 through 13 years. Based on a
(Page
6-21) reference weight of 40 kg (Table 1-3) for boys aged
9 through 13 years, their EAR is 200 mg (8.3 mmol)/day.
| EAR
for Boys |
9
through 13 years |
200 mg
(8.3 mmol)/day |
Based on the 1994 CSFII magnesium
intake data adjusted for day-to-day variation (Nusser et al.,
1996), the tenth percentile of intake for boys aged 9 through 13
years is 181 mg (7.5 mmol)/day, and the twenty-fifth percentile
intake is 216 mg (9 mmol)/day (see Appendix D). Thus the EAR of
200 mg (8.3 mmol)/day for boys aged 9 through 13 years would fall
between the tenth and twenty-fifth percentile of intake in this
age category.
EAR Summary: Ages 9 through 13 Years, Girls
Because the protein intake of the
younger girls in the Andon et al. (1996) study was not indicated,
it is possible that one of the reasons that most of the girls
were in positive magnesium balance on the 176 mg (7.3 mmol)/day
intake was their higher intake of dietary protein. The second
possible reason is their younger age and body size. In the
absence of additional data and assuming a protein intake of 50
g/day, the EAR for girls aged 9 through 13 years is 5 mg (0.21
mmol)/kg/day, based primarily on the Andon et al. (1996) study,
in which all girls who consumed this level or less were in
positive balance. Their average weights were 41 kg (90 lb), and
few had yet started menarche. In the Greger et al. study (1979),
the average weight was 52.5 kg (115.7 lb), and 6 of the 11 girls
had already started menarche. Their requirements reflect a
greater lean body mass. Thus, based on the reference weight of 40
kg (Table 1-3) for girls aged 9 through 13 years, their EAR is
200 mg (8.3 mmol)/day.
| Ear
for Girls |
9
through 13 years |
200 mg
(8.3 mmol)/day |
Based on the 1994 CSFII intake
data and adjusted for day-to-day variation (Nusser et al., 1996),
the twenty-fifth percentile magnesium intake for girls aged 9
through 13 years is 194 mg (8.1 mmol), and the median intake is
224 mg (9.3 mmol)/day (see Appendix D). Thus, the magnesium EAR
of 200 mg (8.1 mmol)/day would be between the twenty-fifth
percentile and the median intake for girls aged 9 through 13
years.
Determination of the RDA for Magnesium: Ages 9 through 13
Years
The variance in requirements
cannot be determined from the data available. Thus, a CV of
approximately 10 percent is assumed for each EAR. This results in
a RDA of 240 mg (10 mmol)/day for both boys and girls aged 9
through 13 years.
| RDA
for Boys |
9
through 13 years |
240 mg
(10 mmol)/day |
| RDA
for Girls |
9
through 13 years |
240 mg
(10 mmol)/day |
(Page 6-22)
EAR Summary: Ages 14 through 18 Years
Given the available data, it
seems appropriate to conclude that for older adolescents, the
average magnesium requirement is greater than 5 mg (0.21
mmol)/kg/day. The average additional magnesium intake
necessary to result in a net retention of about 8 mg (0.33
mmol)/day is about 16 mg (0.67 mmol) or 0.3 mg (0.12 mmol)/kg/day
for a 55 kg adolescent based on an assumed absorption rate of 40
percent (range 30 to 50 percent) (Abrams et al., in press). Less
than 5 mg (0.21 mmol)/kg/day of magnesium resulted in negative
balances in all boys when they consumed the lower protein intake
in the Schwartz et al. (1973) study and in most of the older
girls in the Greger et al. (1978) study. In the 2-year Schwartz
et al. (1973) study, even for subjects on the high protein diet,
average magnesium retention was 6 and 15 mg (0.25 and 0.63
mmol)/day for each year, respectively, at the 240 mg (10
mmol)/day intake level. Thus, the EAR is estimated to be 5.3 mg
(0.22 mmol)/kg/day in 14- through 18- year-old boys and girls,
given that the highest average level provided in any of the five
long- term balance studies (Andon et al., 1996; Greger et al.,
1978, 1979; Schwartz et al., 1973, Sojka et al., in press) was
5.6 mg (0.23 mmol)/kg/day. This resulted in slightly negative
nitrogen balances in the older children studied (Greger et al.,
1978; Schwartz et al., 1973). An average intake of 6.4 mg (0.27
mmol)/kg/day resulted in net positive magnesium retention in the
more recent stable isotope study of Abrams et al. (in press).
Thus, for boys aged 14 through 18 years, with reference weight 64
kg (Table 1-3), the EAR for magnesium is 340 mg (14.2 mmol)/day,
and for girls in this age range with reference weight of 57 kg,
the EAR is 300 mg (12.5 mmol)/day.
| EAR
for Boys |
14
through 18 years |
340 mg
(14.2 mmol)/day |
| EAR
for Girls |
14
through 18 years |
300 mg
(12.5 mmol)/day |
Based on the 1994 CSFII intake
data, adjusted for day-to-day variation (Nusser et al., 1996),
the magnesium EAR of 345 mg (14.4 mmol)/day for boys aged 14
through 18 years was above the median intake of 301 mg (12.5
mmol)/day and below the seventy-fifth percentile of intake, 372
mg (15.5 mmol)/day (see Appendix D). For girls in this age range,
the EAR for magnesium of 300 mg (12.5 mmol)/day was just above
the ninetieth percentile of intake, 296 mg (12.3 mmol)/day.
Determination of the RDA: Ages 14 through 18
Years
The variance in requirements
cannot be determined from the available data. Thus, a CV of
approximately 10 percent is assumed for each EAR. This results in
an RDA for magnesium of 410 mg (17.2 mmol)/day for boys aged 14
through 18 years and an RDA for magnesium of 360 mg (IS. 1
mmol)/day for girls aged 14 through 18 years when rounded.
| RDA
for Boys |
14
through 18 years |
410 mg
(19.5 mmol)/day |
| RDA
for Girls |
14
through 18 years |
360 mg
(17.1 mmol)/day |
(Page 6-23)
Ages 19 through 30 Years
Indicators Used to Set the EAR for Men
Balance Studies. The results of studies that have
looked at magnesium balance in men and women aged 19 through 30
years in various situations are included in Table 6-2. As with
the balance studies conducted in adolescents, no estimates or
measurements of other losses of magnesium (due to dermal, sweat,
etc.) are included. Since few references are available to
estimate these losses, gross balances are presented. In the
controlled diet study (Greger and Baier, 1983), which was
designed to look at the influence of aluminum on mineral
balances, men (25 ± 3 years) were in positive balance on
average intakes of 447 mg (18.6 mmol) from food and dietary
supplements. This represents 6.4 mg (0.27 mmol)/kg/day for these
subjects, but may overestimate requirements as only one level was
given.
Another controlled study, this
one designed to look at the effect of bran on mineral
requirements, was conducted in seven men aged 22 to 32 years
(Schwartz et al., 1986); dietary magnesium intake was 719
± 105 mg (30 ± 4.4 mmol)/day. With one exception,
all subjects were in magnesium equilibrium or positive balance
after 48 days. These levels were thus not low enough to
accurately estimate average requirements. In a year-long study of
men aged 20 to 35 years, subjects kept daily food records for 1
year, and 1-week balance studies were conducted 4 times. The
subjects continued to select their own diet but provided food and
beverage samples for analysis (Lakshmanan et al., 1984). The
assumption in this study was that each subject's average of the 4
weekly balances over the year would be representative of his
typical intake and requirement. The average magnesium intake of
333 ± 120 mg (13.9 ± 5 mmol)/day resulted in a
slight overall average negative balance. Again, since multiple
levels were not evaluated, it is difficult to ascertain actual
requirements from these data; however it appears that the intakes
of five of the nine subjects consuming this average intake of 333
mg (13.9 mmol)/day of magnesium were probably meeting their
needs.
Indicators Used to Set the EAR for Women
Balance Studies. The
results of two balance studies for women in this age range are
also summarized in Table 6-2. The year-long study by Lakshmanan
and coworkers (1984) included young women (20 to 35 years) on
self-selected diets, with duplicates of week-long intakes
analyzed 4 times during the year. The average magnesium intake of
this age group of women was 239 ± 80 mg (10 ± 3.3
mmol); this intake resulted in positive magnesium balance or
equilibrium in three of the eight subjects. A controlled food
intake study in Germany tested a low-phytate barley fiber added
to two levels of dietary protein to determine the effects of the
fiber and protein on mineral balances (Wisker et al., 1991).
Magnesium intake of 243 to 252 mg (10. 1 to 10.5 mmol)/day
(reported to be on the average 4.3 mg [0. 18 mmol]/kg/day) in
women resulted in the 12 subjects being close to equilibrium on
the low- fiber, high-protein diet but not as close on the
high-fiber, low-protein diet.
(Page 6-24)

(Page 6-25)
EAR Summary: Ages 19 through 30 Years, Men
Based primarily on the study of Lakshmanan et al. (1984) and
the others cited above, the EAR for magnesium for males aged 19
through 30 years is estimated to be 330 mg (13.8 mmol)/day. This
is based on the assumption that the best current indicator of
adequacy, given the lack of supporting data for other outcomes,
is for an individual to maintain total body magnesium over time
as opposed to being in negative magnesium balance. The absence of
confirmatory research providing causal relationships in human
studies between magnesium intake and risk of cardiovascular
disease precludes use of markers for cardiovascular disease in
this age group at this time. This EAR also reflects the lack of
data to support the concept that there is a need for continued
accretion of magnesium during this life-stage.
| EAR
for Men |
19
through 30 years |
330 mg
(13.8 mmol)/day |
Based on the 1994 CSFII intake
data and adjusted for day-to-day variation (Nusser et al., 1996),
the median magnesium intake for men aged 19 to 30 years is 331
(13.8 mmol)/day (see Appendix D), which is approximately the same
magnesium intake as the EAR of 330 mg (13.8 mmol)/day.
EAR Summary: Ages 19 through 30 Years, Women
Based on the studies described
above, and primarily on the overall negative balances of the
Lakshmanan et al. (1984) study, which found average magnesium
intakes of 239 mg (10 mmol)/day, the EAR for women aged 19
through 30 years is estimated to be above 239 mg (10.0 mmol)/day.
The Wisker et al. (1991) study, at a higher intake, had somewhat
more positive balances on a slightly higher overall intake of 255
mg (10.6 mmol)/day. This EAR is also based on the assumption that
the best current indicator of adequacy, given the lack of
supporting data for other outcomes, is for an individual to
maintain total body magnesium over time as opposed to being in
negative magnesium balance. This EAR also reflects the lack of
data that there is a specific need for accretion of magnesium
during this period.
| EAR
for Women |
19
through 30 years |
225 mg
(10.6 mmol)/day |
Based on the 1994 CSFII intake
data and adjusted for day-to-day variation (Nusser et al., 1996),
the median magnesium intake for women aged 19 through 30 years is
205 mg (8.5 mmol)/day, and the seventy-fifth percentile of
magnesium intake is 250 mg (10.4 mmol)/day (see Appendix D),
which is slightly below the EAR of 255 mg (10.6 mmol)/day.
Determination of the RDA: Ages 19 through 30
Years
The variance in requirements
cannot be determined from the data available for either men or
women. Thus, a CV of 10 percent is assumed in both cases. This
results in an RDA for magnesium in men aged 19 through 30 years
of 400 mg (16.7 mmol)/day, and for women aged 19 through 30 years
of 310 mg (12.9 mmol)/day.
(Page
6-26)
| RDA
for Men |
19
through 30 years |
400 mg
(16.7 mmol)/day |
| RDA
for Women |
19
through 30 years |
310 mg
(12.9 mmol)/day |
Ages 31 through 50 Years
Indicators Used to Set the
EAR in Men
Balance Studies. The
results of five balance studies in men aged 31 through 50 years,
which met the criteria for inclusion, are shown in Table 6-3. Two
controlled-intake studies which looked at the influence of
dietary oxalate and fiber on mineral balances, included magnesium
balances for men aged 34 to 58 years who were consuming either
high-fiber (Kelsay et al., 1979) or high-oxalate (Kelsay and
Prather, 1983) diets. Intakes ranged from 308 to 356 mg (12.8 to
14.8 mmol)/day. On a low, nondigestible fiber diet (4.9 g/day),
average magnesium balance was positive; but the magnesium intake
was not sufficient on the high-fiber or high-oxalate diets to
maintain magnesium balance. Magnesium balance in male subjects
aged 19 to 64 years given lower magnesium intakes (229 or 258 mg
[9.5 or 10.8 mmol]) at two levels of dietary protein has also
been estimated (Mahalko et al., 1983). Average magnesium balance
at either protein level was at equilibrium for this wider-age-
range group, indicating that, at least based on crude magnesium
balance, dietary intake was near adequacy overall. However, in
another study, magnesium intakes of 240 to 264 mg (10 to 11
mmol)/day resulted in net negative balances (-23 mg [1 mmol]/day
and -2 6 mg [1.1 mmol]/day) in the five subjects studied (Spencer
et al., 1994). Magnesium intakes of 789 to 826 mg (32.9 to 34.4
mmol)/day resulted in positive balances in these same subjects.
Finally, in a year-long study of magnesium intakes by individuals
on self-selected diets with periodic measurements of balance,
average intake of seven male subjects aged 35 to 53 years, was
310 ± 88 mg (12.9 ± 3.7 mmol)/day (Lakshmanan et
al., 1984). The individual averages of the four 1-week balance
periods during the year resulted in a group mean negative
magnesium balance, although three subjects had average positive
balances or were in equilibrium and four were in negative
balance.
Indicators Used to Set the EAR in Women
Balance Studies. The
only study in females for this age group was part of the
year-long study of dietary intakes of men and women, which
included an estimation of magnesium balance in 10 women aged 25
to 53 years (Lakshmanan et al., 1984). The average magnesium
intake was 231 ± 80 mg (9.6 ± 3.3 mmol)/day. Four
subjects were in positive or zero balance, while six were in
negative balance, which suggests that 231 mg (9.6 mmol)/day is
probably less than is necessary to prevent magnesium loss in 50
percent of women aged 31 through 50 years. Thus, the average
requirement for women aged 31 through 50 years appears to be
somewhat higher than 231 mg (9.6 mmol)/day.
(Page
6-27)
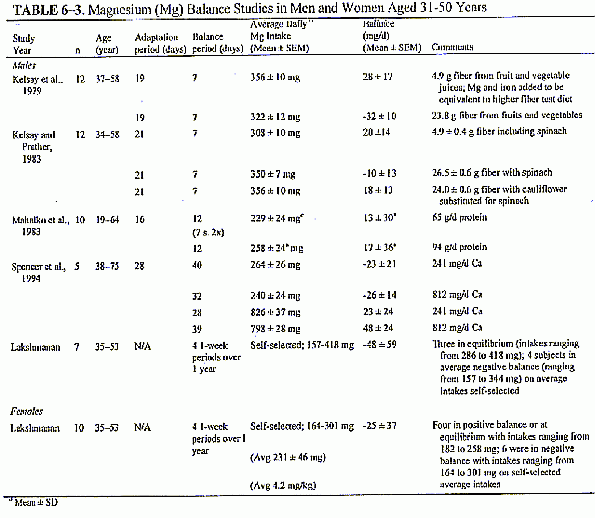
(Page 6-28)
EAR Summary: Ages 31 through 50 years, Men
Based on the studies described
above, the average requirement for males aged 31 through 50 years
does not appear to differ substantially from that of men aged 19
through 30 years. However, there are more instances of negative
balance in the intake range of 300 to 350 mg (12.5 to 14.6
mmol)/day in the older subjects studied. The EAR is thus set at
350 mg (14.6 mmol)/day, with the expectation that with age,
consumption of diets with higher fiber content increases. Since
the two studies in which lower magnesium levels were consumed
resulted in predominantly negative balances (Mahalko et al.,
1983; Spencer et al., 1994), the data of the Lakshmanan et al.
study (1984) raise a concern: the self-selected magnesium intake
of the older men was more than 10 percent below that of the
younger men in the same study.
This EAR is also based on the
assumption that the best current indicator of adequacy, given
lack of supporting data for other outcomes, is for an individual
to maintain total body magnesium over time as opposed to being in
negative magnesium balance. The observed change from average
negative magnesium retention to positive retention or vice versa
caused by changes in other factors in the diet (for example,
fiber, protein) rather than the level of magnesium consumed
provides two perspectives: (1) it gives some assurance that the
dietary level being evaluated is within the range of the average
requirement, and (2) it gives an indication of the many factors
that affect magnesium requirements as evaluated by balance
studies. Thus, it is the combination of balance studies that
provides some assurance that the estimates of average
requirements derived are of value as dietary reference
intakes.
| EAR
for Men |
31
through 50 years |
350 mg
(14.6 mmol)/day |
Based on the 1994 CSFII magnesium
intake data and adjusted for day-to-day variation (Nusser et al.,
1996), the EAR of 350 mg (14.6 mmol)/day for men aged 31 through
50 years fell between the median magnesium intake of 327 mg (13.6
mmol)/day and the seventy-fifth percentile of magnesium intake of
397 mg (16.5 mmol)/day (see Appendix D).
EAR Summary: Ages 31 through 50 Years, Women
Based on the one study described
above in which women aged 25 to 53 years were predominantly in
negative magnesium balance at an average intake of 231 mg (9.6
mmol)/day (Lakshmanan et al., 1984), and comparing the
information on younger women and on men, the EAR for women aged
31 through 50 years is estimated to be 265 mg (11.0 mmol)/day.
This is quite close to the EAR for younger women, which was based
on data from the same study. However, two pieces of evidence lead
to the conclusion that the EAR is somewhat greater for this age
group. Since renal function is critical to maintenance of
magnesium status, and it has been shown to decline with age, it
follows that an increased EAR is warranted. Also, a higher
percentage of the older women were in negative balance compared
to the younger women. This was also demonstrated when comparing
the older men (31 through 50 years) with the younger men (19
through 30 years). This EAR is based on the assumption that the
best current indicator of adequacy, given lack of supporting data
for other outcomes, is for an individual to maintain total body
magnesium over time.
(Page
6-29)
| EAR
for Women |
31
through 50 years |
265 mg
(11.0 mmol)/day |
Based on the 1994 CSFII intake
data and adjusted for day-to-day variation (Nusser et al.,
(1996), the median magnesium intake of women aged 31 through 50
years was 229 mg (9.5 mmol)/day (see Appendix D). This is below
the EAR of 265 mg (11.0 mmol). The seventy- fifth percentile of
intake was 277 mg (11.5 mmol)/day, which is above the EAR.
Determination of the RDA: Ages 31 through 50
Years
The variance in requirements
cannot be determined from the available data for either men or
women. Thus, a CV of 10 percent is assumed for both cases. This
results in an RDA for men aged 31 through 50 years for magnesium
of 420 mg (17.5 mmol) and for women, 320 mg (13.3 mmol)/day.
| RDA
for Men |
31
through 50 years |
420 mg
(17.5 mmol)/day |
| RDA
for Women |
31
through 50 years |
320 mg
(13.3 mmol)/day |
Ages 51 through 70 Years
Indicators Used to Set the EAR for Men
Balance Studies. The
results of five balance studies for men aged 51 through 70 years
are shown in Table 6-4. Balance studies cited in Table 6-3 (aged
31 through 50 years) by Kelsay et al. (1979), Kelsay and Prather
(1983), Mahalko et al. (1983), and Spencer et al. (1994) included
some male subjects in this age range, so these data are also
included in this age group. Schwartz et al. (1984) assessed
magnesium balance in eight males, mean age 53 ± 5
years and mean weight 67 ± 14 kg (148 ± 31 lb). A
positive magnesium balance was found in the men who consumed an
average intake of 381 mg (15.9 mmol)/day of magnesium (5.9 mg or
0.25 mmol/kg/day on the average).
Indicators Used to Set the EAR for Women
No studies have been reported in women in this age group.
EAR Summary: Ages 51 through 70 Years, Men
A mean daily magnesium intake of
3 81 mg (15.9 mmol) maintained balance in all eight subjects in
the Schwartz et al. (1984) study, following a 30-day adaptation
period. This would indicate that, in the absence of other studies
that might demonstrate different results, the EAR should be less
than 380 mg (15.8 mmol) in order to prevent magnesium loss. This
study, along with those discussed above in the other adult age
groups, indicates that the EAR can be expected to be somewhere
between 330 and 380 mg (13.8 and 15.8 mmol)/day. Given the lower
body weights in the Schwartz et al. (1984) study compared with
those in the younger age groups, the magnesium intakes per kg
body weight are greater (approximately (Page
6-30) 5.9 mg [0.25 mmol]/kg/day). Because the data for the
age group 31 through 50 years reflect more instances of negative
balance when dietary magnesium intakes were in the range of 300
to 350 mg (12.5 to 14.6 mmol)/day, it is appropriate to estimate
the EAR to be 350 mg (14.6 mmol)/day, particularly in light of
the importance of renal function to maintaining magnesium
homeostasis. This EAR is based on the assumption that the best
current indicator of adequacy, given lack of supporting data for
other outcomes, is to maintain total body magnesium over
time.
Based on the 1994 CSFII intake
data and adjusted for day-to-day variation (Nusser et al., 1996),
the median intake of magnesium for men aged 51 through 70 years
is 295 mg (12.3) mmol)/day, and the seventy-fifth percentile of
intake was 362 mg (15.1 mmol)/day (see Appendix D), which is
slightly above the EAR of 350 mg (14.6 mmol)/day.
EAR Summary: Ages 51 to 70 Years, Women
Because there are no studies in
women in this age group, the EAR is based on the one study
described in women aged 31 through 50 and on comparisons of the
information in younger women and in men. There is no basis on
which to change the EAR for this age group from that for women
aged 31 through 50 years, which is estimated to be 265 mg (11.0
mmol)/day, other than a concern about the possible decline in
renal function associated with aging. However, since an
adjustment for declining renal function was included in the
estimate of the 31 - through 50-year-old women, no further
adjustment is needed for this age group. Thus, the EAR is also
265 mg (11.0 mmol)/day for women aged 51 through 70 years. This
EAR is also based on the assumption that the best current
indicator of adequacy, given lack of supporting data for other
outcomes, is to maintain total body magnesium over time.
| EAR
for Women |
51
through 70 years |
265 mg
(11.0 mmol)/day |
Based on the 1994 CSFII intake
data and adjusted for day-to-day variation (Nusser et al., 1996),
the median intake of magnesium for women aged 51 through 70 years
is 230 mg (9.6 mmol)/day, and the seventy-fifth percentile of
intake is 276 mg (11.5 mmol)/day (see Appendix D), which is
slightly above the EAR of 265 mg (11.0 mmol)/day for women in
this age range.
Determination of the RDA: Ages 51 through 70
Years
The variance in requirements
cannot be determined from the data available for either men or
women aged 51 through 70 years. Thus, a CV of 10 percent is
assumed for both cases, This results in an RDA for men aged 51
through 70 years of approximately 420 mg (17.5 mmol)/day, and for
women, 320 mg (13.3 mmol)/day.
| RDA
for Men |
51
through 70 years |
420 mg
(17.5 mmol)/day |
| RDA
for Women |
51
through 70 years |
320 mg
(13.3 mmol)/day |
| |
|
|
| EAR
for Men |
51
through 70 years |
350 mg
(14.6 mmol)/day |
(Page 6-31)
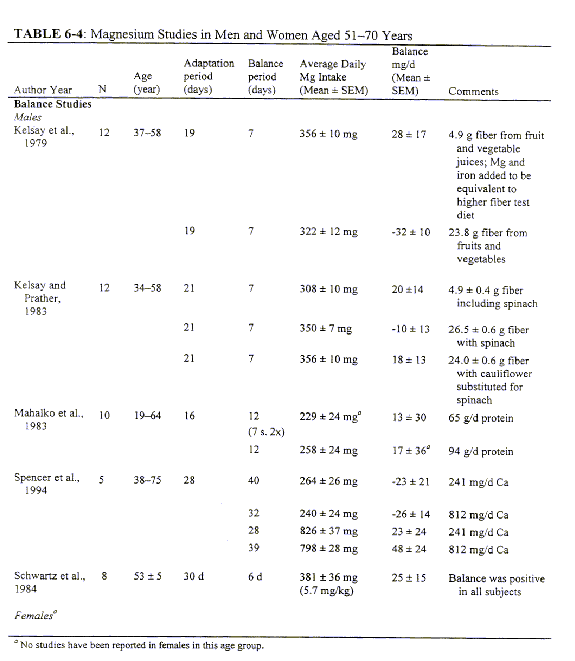
(Page
6-32)
Ages > 70 Years
Indicators Used to Set the EAR
Studies in this age group have aggregated data from both men
and women, and therefore the requirements will be considered
together. The greater numbers of individuals with chronic
diseases in this population, and the comparative lack of research
studies carried out in healthy free-living individuals in this
age category, make estimation of requirements problematic.
Balance Studies. No magnesium balance studies that
meet the criteria previously described have been reported in
subjects over 70 years of age.
Magnesium Tolerance
Tests. One study with 36 healthy elderly subjects (8 males
and 28 females) 65 years of age and older (average age 73
± 6 years), used magnesium tolerance testing as the
indicator of adequacy (Gullestad et al., 1994). The self-selected
dietary magnesium intake was estimated from food frequency
questionnaires to be 380 ± 94 mg (15.8 ± 3.9
mmol)/day in the males and 300 ± 61 mg (12.5 ± 2.5
mmol)/day in the females. When corrected for body weight, this
intake was similar in both sexes, 5.1 mg (0.21 mmol)/kg/day.
Magnesium retention from the load given was 28 ± 16
percent in the elderly and was significantly greater than the 3.6
percent retention in a reference group of 53 subjects aged 55
± 12 years. However, no correlation was seen between
estimated magnesium intake and magnesium retention from the load
given. These data do not provide convincing evidence on which to
base an estimated requirement.
Intracellular Studies.
Intracellular magnesium was assessed in two studies in elderly
subjects. Total red blood cell magnesium was measured in 381
institutionalized, elderly subjects aged 80 ± 9.5 years
(Touitou et al., 1987). The mean dietary magnesium intake was 240
mg (10 mmol)/day. Abnormally low blood cell magnesium was found
in 21 percent of the subjects (about 10 percent had low plasma
magnesium concentrations as well). A large number of these
subjects had conditions and/or had been on medications that
contributed to the apparent poor magnesium status. After
excluding these factors, the prevalence of low red blood cell
magnesium in the remaining 198 subjects was not significantly
different (19 percent). Again, no correlation was found between
dietary intake and red blood cell magnesium values. In another
study, red blood cell magnesium values in 12 nonobese elderly
subjects (6 males, 6 females) aged 78 ± 2 years were
compared with those of 25 young healthy subjects (13 males, 12
females) aged 36 ± 0.4 years (Paolisso et al., 1992).
Self-selected dietary magnesium intakes of both groups were
estimated to be 311 ± 21 mg (13.0 ± 0.9 mmol)/day.
Red blood cell magnesium levels in the elderly were 1.86
mmol/liter (4.5 mg/dl), which was significantly less than that of
the control subjects, with average levels of 2.18 mmol/liter (5.2
mg/dl). Magnesium therapy in the elderly resulted in a rise in
red blood cell magnesium and an increase in insulin secretion and
action, which suggests that the low red blood cell magnesium was
physiologically relevant in this population.
(Page 6-33)
EAR Summary: Ages > 70 Years
Because no balance studies
meeting appropriate criteria are available, other possible
indicators of magnesium requirements for this age group were
reviewed. However, no conclusive studies were found. The methods
used in the studies of magnesium tolerance testing and
intracellular magnesium discussed above have yet to be validated
sufficiently to serve as the basis for estimating average
requirements.
The reported magnesium intakes
from the three available studies using these methodologies,
however, are consistent with balance studies in younger age
groups. The study by Gullestad et al. (1994), using magnesium
tolerance testing, found an estimated average dietary magnesium
intake in males of 380 mg (15.8 mmol)/day and 300 mg (12.5
mmol)/day in females. The study of intracellular magnesium by
Touitou and coworkers (1987) suggests that the average
requirement would be approximately 240 mg [10 mmol]/day). The
study by Paolisso and colleagues (1992) found that in elderly
subjects, an average dietary magnesium intake of 311 mg (13.0
mmol)/day was accompanied by a lower mean red blood cell
magnesium concentration, which was not found in younger
controls.
Given the uncertainty in the
above methods, and the lack of balance data in healthy, elderly
individuals to support the intake levels that may appear to be
warranted based on the above analysis, estimates of magnesium
requirements are suggested to remain at the level established for
the other older adult age groups. These estimates would be within
the range identified above as estimating the average requirement
for elderly. It must be remembered, though, that urinary
magnesium excretion has been shown to increase with age (Lowik et
al., 1993, Martin, .1990), indicating a decrease in renal
function.
| EAR
for Men |
>
70 years |
350 mg
(14.6 mmol)/day |
| EAR
for Women |
>
70 years |
265 mg
(11.0 mmol)/day |
Based on the 1994 CSFII intake
data and adjusted for day-to-day variation (Nusser et al., 1996),
the median magnesium intake for men aged > 70 years is 274 mg
(11.4 mmol)/day (see Appendix D). The EAR of 350 mg (14.6
mmol)/day falls between the seventy-fifth percentile of magnesium
intake of 334 mg (13.9 mmol)/day and the ninetieth percentile of
intake of 394 mg (16.4 mmol)/day. For women in this same age
range, the median magnesium intake is 205 mg (8.5 mmol)/day. As
for men aged > 70 years, the magnesium EAR for women of 265 mg
(11.0 mmol)/day falls between the seventy-fifth percentile of
intake of 248 mg (10.3 mmol)/day and the ninetieth percentile of
intake of 290 mg (12.1 mmol)/day.
Determination of the RDA for Magnesium: Ages > 70
Years
The variance in requirements
could not be determined from the available data for either men or
women aged > 70 years. Thus, a CV of 10 percent is assumed for
the > 70 years age group. This results in an RDA for men aged
> 70 years for magnesium of approximately 420 mg (17.5
mmol)/day and for women aged > 70 years of 320 mg (13.3
mmol)/day.
(Page 6-34)
| RDA
for Men |
>
70 years |
420 mg
(17.5 mmol)/day |
| RDA
for Women |
>
70 years |
320 mg
(13.3 mmol)/day |
Pregnancy
Indicators Used to Set the EAR
Serum Magnesium Concentrations. Because serum
magnesium concentration is reduced during pregnancy (Kurzel,
1991; Weissberg et al., 1992), the use of magnesium sulfate as a
tocolytic agent has led some investigators to study the possible
role of magnesium status in determining pregnancy and infant
outcome, including the incidence of preterm labor and
pregnancy-induced hypertension and fetal growth retardation,
mental retardation, and cerebral palsy in the newborn (Conradt et
al., 1984; Rudnicki et al., 1991; Schendel et al., 1996).
However, the reduction in serum magnesium concentration during
pregnancy is thought to be due, in part, to hemodilution, and
this decrease parallels the decrease seen in serum protein
(Seydoux et al., 1992). Serum ionized magnesium has also been
reported to decrease late in pregnancy (Bardicef et al., 1995;
Handwerker et al., 1996). Therefore, serum magnesium
concentrations do not appear to be adequate indicators of
magnesium status.
Intracellular Magnesium.
Inconsistent findings have been reported on changes in lymphocyte
magnesium concentrations during pregnancy. Some investigators
report no change (Seydoux et al., 1992), while others find
intracellular magnesium depletion (Bardicef et al., 1995). Such
indicators of magnesium status have not been adequately assessed
during pregnancy and thus are not used here as the basis for
determining requirements.
Balance Studies. Few
magnesium balance studies have been performed in pregnant
subjects. One study of 48 magnesium balances conducted in 10
subjects for 7-day periods at various stages of pregnancy has
been reported (Ashe et al., 1979). The balances, which were not
carried out in a metabolic unit setting, demonstrated an average
negative magnesium balance of -40 mg (-1.7 mmol)/day on a mean
daily magnesium intake of 269 ± 55 mg (11.2 ± 2.3
mmol) (Ashe et al., 1979).
Magnesium Tolerance
Tests. Parenteral magnesium load tests in postpartum
American and Thai women have been conducted to evaluate the
methodology (Caddell et al., 1973, 1975). In 185 American women,
the mean magnesium retention was 51 percent. Higher magnesium
retention was associated with lower serum magnesium
concentrations as well as with diets low in magnesium-rich foods
(Caddell et al., 1975). In Thai women, who consumed a diet
containing more magnesium-rich foods, a mean retention of 23
percent was observed (Caddell et al., 1973).
Pregnancy Outcome.
Several cross-sectional studies have investigated whether
magnesium status is altered in women with gestational diabetes,
preterm labor, pregnancy- induced hypertension, or preeclampsia
(Bardicef, et al., 1995; Kurzel, 1991; Seydoux et al., 1992;
Weissberg et al., 1992). The results of these studies are not
consistent, possibly due to (Page
6-35) the control groups that were used or the inability
to distinguish whether altered magnesium status precedes the
outcome or the outcome influences magnesium status.
In one cross-sectional study,
lower serum magnesium concentrations were observed in women
during preterm labor (n = 71) compared with normal pregnant women
in labor (n = 128) (Kurzel, 1991). Although this study
found no difference in serum magnesium concentrations between the
two groups, others have observed reduced serum magnesium during
labor (Weissberg et al., 1992). Therefore, it is not clear
whether hypomagnesemia induces uterine irritability and leads to
preterm labor or whether labor results in a reduction in serum
magnesium concentration.
Longitudinal studies have an
advantage over cross-sectional studies in that changes in
magnesium intake or status can be determined prior to knowing the
final outcome of pregnancy. A prospective observational study of
965 women who were followed from 30 weeks gestation found no
effect of magnesium intake on birthweight, as determined by a
self- administered questionnaire and a structured interview
(Skajaa et al., 1991). The mean reported daily magnesium intake
was relatively high at 445 mg; (18.5 mmol) for the entire
population, with a 95 percent range of 256 to 631 mg (10.7 to
26.3 mmol). The subgroups of women who gave birth to a
small-for-gestational age infant, had preterm labor, or who later
developed preeclampsia all had similar mean magnesium intakes and
serum magnesium concentrations during their third trimester
compared with women who had normal pregnancies. There were no
differences in tissue magnesium concentrations determined from
either abdominal rectus or myometrial muscle biopsies among women
delivering by cesarean section because of intrauterine growth
retardation (n = 5), preeclampsia (n = 12), or labor
difficulties (n = 14). The results of this study appear
to indicate that intrauterine growth retardation, preterm labor,
and preeclampsia are not associated with daily magnesium intakes
around 445 mg (18.5 mmol) or with serum magnesium concentrations
at approximately 30 weeks gestation; and in a small number of
women with adverse outcomes, tissue concentrations of magnesium
were not abnormal at the time of delivery (Skajaa et al.,
1991).
A retrospective study by Conradt
and coworkers (1984) found that magnesium supplementation had a
beneficial effect on pregnancy outcome. Pregnancy outcomes were
studied during a period when the practice of magnesium
supplementation changed. Women with high-risk pregnancies were
routinely supplemented with 36 to 72 mg (1.5 to 3 mmol)/day
magnesium throughout the study (n = 660), whereas during the
previous 2 years women were routinely supplemented with 360 to
480 mg (15 to 20 mmol)/day magnesium (n = 264). Unfortunately,
the study groups were not randomized, and baseline estimates of
magnesium intakes were not determined. Pregnancy outcomes among
these two groups were compared with outcomes among high-risk
women not receiving magnesium supplements (n = 4,023). The
results showed that women receiving either level of magnesium did
not develop pregnancy-induced hypertension and preeclampsia;
among those not receiving supplements, 97 cases (2 percent)
occurred. Women with the higher intake of magnesium had a lower
incidence of intrauterine growth retardation than did women with
the lower intake.
Three prospective trials of
magnesium supplementation during pregnancy have been conducted
(Table 6-5). One study was a double-blind, randomized, controlled
trial (Sibai et al., 1989), one was a quasi-randomized trial
(group assignment determined by mother's date of birth) (Spatling
and Spatling, 1988), and the randomization method of the other
was not (Page
6-36) clear (Kuti et al., 1981). In the first two studies,
women were supplemented with magnesium aspartate-hydrochloride
containing 365 mg (15.2 mmol)/day of magnesium. In the third
study, women were supplemented with magnesium citrate, which
provided a daily magnesium average intake of 170 to 340 mg (7.1
to 14.2 mmol). Women were categorized into three levels of total
magnesium consumed throughout the pregnancy. Daily magnesium
intake from supplements was estimated to be 127 to 202 mg (5.3 to
8.4 mmol) for the category with the highest level of magnesium
consumed (Table 6-5).
Baseline magnesium intake was not
reported in any of the studies. The mean daily magnesium intake
for women in Hungary at the time the study by Kuti and coworkers
(1981) was conducted was 330 mg (13.8 mmol). Daily magnesium
intakes by pregnant women in the United States, where the study
by Sibai and coworkers was completed, ranged from 158 to 259 mg
(6.6 to 10.8 mmol) (Franz, 1987). Based on these baseline
estimates of dietary magnesium intake and the amounts provided in
the above trials, total magnesium intakes in the supplemented
groups would have ranged from 420 to 625 mg (17.5 to 26 mmol).
The results of magnesium supplementation trials indicate that the
incidence of preeclampsia and intrauterine growth retardation was
not affected by magnesium supplementation in two studies, the
incidence of preterm delivery decreased in only one of three
studies, and preterm labor was less frequent in one of two
studies (see Table 6-5).
Accretion Rate during
Pregnancy. The increase in body weight caused by lean tissue
accretion during pregnancy is expected to result in a greater
requirement for magnesium if there are no pregnancy-induced
increases in intestinal absorption and renal reabsorption. Data
are not available on accretion of magnesium in lean tissue during
pregnancy, but this accretion can be estimated (see following
section). Given that fat-free body mass contains possible to
determine the amount necessary for accretion for an appropriate
weight gain.
EAR Summary for Pregnancy
Inconsistent findings on the
effect of magnesium supplementation on pregnancy outcome make it
difficult to determine whether magnesium intakes greater than
those recommended for non-pregnant women are beneficial. In
addition, there are no data indicating that magnesium is
conserved during pregnancy or intestinal absorption is increased.
The gain in weight associated with pregnancy alone may result in
a greater requirement for magnesium.
The EAR for pregnancy is set at
an additional 35 mg (1.5 mmol)/day. This additional requirement
is based on the following assumptions:
- Appropriate added lean body mass (LBM) is 6 to 9 kg with a
midpoint of 7.5 kg (IOM, 1991).
- The magnesium content of 1 kg of LBM is 470 mg (19.6 mmol)
(Widdowson and Dickerson, 1964).
- The adjustment factor for a bioavailability of 40 percent
(Abrams et al., 1997) is 2.5.
Calculation: (7-5 kg /270 days) X 470 mg/kg X 2.5 = 33 mg/day
(rounded up to 35) This value is to be added to the EAR for the
woman's age group.
(Page 6-37)
(Page 6-38)
| EAR
for Pregnancy |
14
through 18 years |
+35 mg
(1.5 mmol)/day |
Based on the 1994 CSFII intake
data for 33 pregnant women and adjusted for day-to-day variation
(Nusser et al., 1996), the median magnesium intake of pregnant
women was 292 mg (12.2 mmol)/day, and the seventy-fifth
percentile of intake was 332 mg (13.8 mmol)/day (see Appendix D).
The EAR of 290 mg (12.1 mmol)/day for pregnant women aged 19
through 3 0 years and the EAR of 300 mg (12.7 mmol)/day for
pregnant women aged 31 through 50 would fall close to the median
of magnesium intake. The seventy-fifth percentile of intake, 332
mg (15.3 mmol)/day, is near the magnesium EAR of 335 mg (15.2
mmol)/day for pregnant women aged 14 through 18 years, about 470
mg (19.6 mmol) of magnesium/kg (Widdowson and Dickerson, 1964),
it is
Determination of the RDA: Pregnancy
The variance in requirements
cannot be determined from the available data for pregnant women.
Thus, a CV of 10 percent is assumed. This results in an increase
in the RDA for pregnancy for magnesium is as follows:
|
Pregnancy |
EAR |
RDA |
| 14
through 18 years |
335 mg
(14.0 mmol)/day |
400
(16.7 mmol)/day |
| 19
through 30 years |
290 mg
(12.7 mmol)/day |
350
(15.0 mmol)/day |
| 31
through 50 years |
300 mg
(12.7 mmol)/day |
360
(15.0 mmol)/day |
Special Considerations
Diabetes Mellitus.
Infants of mothers with Type I insulin-dependent diabetes
mellitus are at risk of hypocalcemia and hypomagnesemia, possibly
due to magnesium deficiency in the mother (Mimouni et al., 1986;
Tsang et al., 1976). Lower intracellular magnesium concentrations
have been recently reported in women with gestational diabetes
(Bardicef et al., 1995). It is not known whether this is a
sequellae of the condition or a factor in its causation.
Pregnant Adolescents, Multiparous Births. A
prospective study of 53 nulliparous teenagers found no difference
in serum or erythrocyte magnesium concentrations between those
pregnant adolescents who developed pregnancy-induced hypertension
and those who had normal term deliveries, with both groups having
decreasing concentrations of magnesium over gestation (Boston et
al., 1989). However, Caddell and coworkers (1975) found a greater
renal retention of a parenteral load of magnesium in pregnant
adolescents and women with twin pregnancies, suggesting that
magnesium requirements during these periods may be increased.
(Page 6-39)
Lactation
Indicators Used to Set the EAR
Human Milk Content. The concentration of magnesium in
human milk averages between
25 to 35 mg/liter (1.0 to 1.5
mmol/liter) and is not influenced by the mother's magnesium
intake (Moser et al., 1983, 1988). Assuming a milk production of
780 ml/day, a lactating woman may secrete from 9 to 26 mg (0.4 to
1.1 mmol)/day of magnesium in her milk (Allen et al., 1991).
Despite the secretion of
magnesium in milk during lactation, plasma and erthyrocyte
magnesium concentrations do not differ between lactating and
nonlactating women at daily magnesium intakes of approximately
250 mg (10.4 mmol) (Moser et al., 1983), and milk concentrations
do not change throughout lactation (Dewey et al., 1984; Moser et
al., 1983; Rajalakshmi and Srikantia, 1980).
Balance Studies. A
magnesium balance study in six lactating women, six nonlactating
postpartum women, and seven women who were never pregnant found
lower urinary magnesium concentrations in lactating women
compared with women who were never pregnant (Dengel et al.,
1994). A positive magnesium balance of 20 mg (0.84 mmol)/day was
reported in lactating women consuming a daily magnesium intake of
217 mg (9 mmol). However, there was only a 5-day adaptation
period, and although the women appeared to conserve magnesium,
the small number of subjects may have lead to an insufficient
ability to detect a difference. Whether the increased bone
resorption that occurs during lactation contributes to the
magnesium pool available for milk production, or whether renal
conservation is sufficient to meet the increased need, is
unknown. Urinary magnesium concentrations in the lactating women
were similar to those of nonlactating postpartum women; however,
the 24-hour urinary magnesium losses in these lactating women
were similar to urinary losses in women determined to be
magnesium depleted based on the results of magnesium loading
tests (Caddell et al., 1975). Although this study found lower
urinary magnesium excretion in lactating women consuming an
estimated daily average magnesium intake of 217 mg (9 mmol),
another study found no difference in urinary magnesium
concentrations between lactating and never-pregnant women who
consumed higher average daily intakes of magnesium, around 270 mg
(11.3 mmol) (Klein et al., 1995).
EAR Summary for Lactation
Currently, no consistent evidence
exists to support an increased requirement for dietary magnesium
during lactation. It appears that decreased urinary excretion of
magnesium and increased bone resorption during lactation may
provide the necessary magnesium for milk production. Therefore,
the EAR and RDA are estimated to be the same as that obtained for
nonlactating women of similar age and body weight.
(Page 6-40)
|
Lactation |
EAR |
RDA |
| 14
through 18 years |
300 mg
(12.5 mmol)/day |
360 mg
(15.0 mmol)/day |
| 19
through 30 years |
255 mg
(11.3 mmol)/day |
310 mg
(13.3 mmol)/day |
| 31
through 50 years |
265 mg
(11.3 mmol)/day |
320 mg
(13.3 mmol)/day |
Based on the 1994 CSFII intake
data and adjusted for day-to-day variation (Nusser et al., 1996),
the median intake of magnesium in 16 lactating women was 3 16 mg
(13.2 mmol)/day; the fifth percentile of intake for the 16 women
(of unspecified age) was 267 mg (11.1 mmol)/day (see Appendix D),
which is slightly above the magnesium EAR for lactating women 19
through 30 years and close to the magnesium EAR of 265 mg (11.3
mmol)/day for lactating women aged 31 through 50 years. The
twenty-fifth percentile intake was 296 mg (12.3 mmol)/day, which
is slightly below the EAR of 300 mg (13.8 mmol)/day for lactating
women aged 14 through 18 years.
Special
Considerations
Mothers Nursing Multiple
Infants. Increased intakes of magnesium during lactation, as
with calcium, should be considered in mothers nursing multiple
infants concurrently. Magnesium requirements may be higher due to
the increased milk production of a mother while nursing multiple
infants. It is not known whether decreased urinary magnesium and
increased maternal bone resorption provide sufficient amounts of
magnesium to meet these increased needs.
TOLERABLE UPPER INTAKE LEVELS
Hazard Identification
Magnesium, when ingested as a naturally-occurring substance in
foods, has not been demonstrated to exert any adverse effects.
However, adverse effects of excess magnesium intake have been
observed with intakes from nonfood sources such as various
magnesium salts used for pharmacologic purposes. Thus, a
Tolerable Upper Intake Level (UL) cannot be based on magnesium
obtained from foods. All reports of adverse effects of excess
magnesium intake concern magnesium taken in addition to that
consumed from food sources. Therefore, for the purposes of this
review, magnesium intake that could result in adverse effects was
from that obtained from its pharmacological use.
The primary initial manifestation
of excessive magnesium intake from nonfood sources is diarrhea
(Mordes and Wacker, 1978; Rude and Singer, 1980). Magnesium has a
well-known cathartic effect and is used pharmacologically for
that purpose (Fine et al., 1991b). The diarrheal effect produced
by pharmacological use of various magnesium salts is an osmotic
effect (Fine et al., 1991b) and may be accompanied by other mild
gastrointestinal effects such as nausea and abdominal cramping
(Bashir et al., 1993; Marken et al., 1989; Ricci et al., 1991).
Osmotic diarrhea has not been reported with normal dietary
intakes of magnesium. Magnesium ingested as a component of food
or food fortificants has not been reported to cause this mild,
osmotic diarrhea even when large amounts are ingested.
(Page 6-41)
Magnesium is absorbed much more
efficiently from the normal concentrations found in the diet than
it is from the higher doses found in nonfood sources (Fine et
al., 1991a). The presence of food likely counteracts the osmotic
effect of the magnesium salts in the gut lumen (Fine et al., 1991
a). In normal individuals, the kidney seems to maintain magnesium
homeostasis over a rather wide range of magnesium intakes. Thus,
hypermagnesemia has not been documented following the intake of
high levels of dietary magnesium in the absence of either
intestinal or renal disease (Mordes and Wacker, 1978).
Hypermagnesemia can occur in
individuals with impaired renal function and is most commonly
associated with the combination of impaired renal function and
excessive intake of nonfood magnesium (for example, as antacids)
(Mordes and Wacker, 1978; Randall et al., 1964). Hypermagnesemia
resulting from impaired renal function and/or intravenous
administration of magnesium can result in more serious
neurological and cardiac symptoms, but elevated serum magnesium
concentrations greater than 2 to 3.5 mmol/liter (4.8 to 8.4
mg/dl) must be attained before onset of these symptoms (Rude and
Singer, 1980). Intakes of nonfood magnesium have rarely been
reported to cause symptomatic hypermagnesemia in individuals with
normal renal function.
Although magnesium supplements
are used (see Table 2-2), comparatively few serious adverse
reactions are reported until high doses are ingested (see data
following). However, some individuals in the populations may be
at risk of a mild, reversible adverse effect (diarrhea) even at
doses from nonfood sources that are easily tolerated by others.
Thus, diarrhea was chosen as the most sensitive toxic
manifestation of excess magnesium intake from nonfood
sources.
It is not known if all magnesium
salts behave similarly in the induction of osmotic diarrhea. In
the absence of evidence to the contrary, it seems prudent to
assume that all dissociable magnesium salts share this property.
Reports of diarrhea associated with magnesium frequently involve
preparations that include aluminum, and therefore a specific
magnesium-associated effect cannot be ascertained.
Large pharmacological doses of
magnesium can clearly result in more serious adverse reactions.
An 8-week-old infant suffered metabolic alkalosis, diarrhea, and
dehydration after receiving large amounts of magnesium oxide
powder on each of two successive days (Bodanszky and Leleiko,
1985). Urakabe et al. (1975) reported that a female adult
suffered from metabolic alkalosis and hypokalemia from the
repeated daily ingestion of 30 g (1,250 mmol) of magnesium oxide.
Several cases of paralytic ileus were encountered in adult
patients who had taken large, cathartic doses of magnesium: in
one case, two bottles of magnesium citrate and several doses of
milk of magnesia, and in the other case, several doses of
magnesium sulfate in a patient with mild renal impairment
(Golzarian et al., 1994). Cardiorespiratory arrest was
encountered in a suicidal patient given 465 g (19.1 mol) of
magnesium sulfate as a cathartic to counteract an intentional
drug overdose (Smilkstein et al., 1988). Deaths from very large
exposures to magnesium in the form of magnesium sulfate or
magnesium oxide have been reported following cardiac arrest,
especially in individuals with renal insufficiency (Randall et
al., 1964; Thatcher and Rock, 1928).
(Page 6-42)
Dose-Response Relationship
Adolescents and Adults: Ages > 9 Years
Data Selection. A review
of the scientific literature revealed relatively few reports that
were useful in establishing a UL for magnesium. Because magnesium
has not been shown to produce any toxic effects when ingested as
a naturally occurring substance in foods, a UL cannot be
established for dietary magnesium at this time. In addition,
studies involving intravenous administration of comparatively
large doses of magnesium used in the treatment of preterm labor,
pregnancy-induced hypertension, or other clinical conditions were
not considered applicable for the derivation of ULs. Based on
limited data described below, a UL can be established for
magnesium from nonfood sources.
Identification of a NOAEL (or
LOAEL) and Critical Endpoint. As the primary initial
manifestation of excessive magnesium intake, diarrhea was
selected as the critical endpoint. The few studies that report
mild diarrhea and other gastrointestinal symptoms from uses of
magnesium salts were reviewed to identify a
No-Observed-Adverse-Effect Level (NOAEL) (or
Lowest-Observed-Adverse-Effect-Level [LOAEL]). Gastrointestinal
symptoms, including diarrhea, developed in 6 of 21 patients (51-
to 70-year-old males and females) receiving long-term magnesium
chloride therapy at levels of 360 mg (15 mmol) of magnesium
(Bashir et al., 1993). Gastrointestinal manifestations developed
in 5 of 25 pregnant women being given 384 mg (16 mmol) of
magnesium as magnesium chloride supplements for the prevention of
preterm delivery, although one patient receiving the placebo
treatment also developed diarrhea (Ricci et al., 1991). Diarrhea
was also noted in 18 of 50 healthy white and black men and women
(aged 31 through 50 years) who were ingesting 470 mg (19.6 mmol)
of magnesium as magnesium oxide daily (Marken et al., 1989).
Levels of fecal output of soluble magnesium and fecal magnesium
concentration were elevated in individuals with diarrhea induced
by 168 to 2,320 mg (7 to 97 mmol) of magnesium as magnesium
hydroxide (Fine et al., 1991b).
However, other studies using
similar or even higher levels of supplemental magnesium reported
no diarrhea or other gastrointestinal complaints. Healthy 18- to
38-year-old males given diets enriched with magnesium oxide at
levels up to 452 mg (18.9 mmol) daily for 6 days did not report
the occurrence of any gastrointestinal symptoms (Altura et al.,
1994). This study of the effect of magnesium-enriched diets on
absorption involved the fortification of foods with magnesium,
which may have different effects from the administration of
magnesium supplements outside the normal diet. Furthermore, no
diarrhea was reported in patients of varying ages receiving an
average of 576 mg (24 mmol)/day of supplemental magnesium as
magnesium oxide in a metabolic balance study for 28 days (Spencer
et al., 1994). Diarrhea or other gastrointestinal complaints were
not observed in patients receiving up to 1,200 mg (50 mmol) of
magnesium in the form of an aluminum-magnesium- hydroxycarbonate
antacid over a 6-week trial period (Nagy et al., 1988). In a
longer-term study, a group of postmenopausal women received daily
supplements of 226 to 678 mg (9.4 to 28.3 mmol) of magnesium as
magnesium hydroxide for 6 months followed by 226 mg (9.4 mmol) of
magnesium for 18 months without any observations of
gastrointestinal complaints (Stendig-Lindberg et al., 1993). A
group of persons with diabetes was supplemented with (Page
6-43) 400 mg (16.7 mmol) of magnesium daily for 8 weeks in
the form of magnesium oxide or magnesium chloride without any
gastrointestinal complications (Nadler et al., 1992). Elderly
subjects supplemented with 372 mg (15.5 mmol) of magnesium daily
over a 4-week period did not report any diarrheal effects or
other gastrointestinal complaints (Paolisso et al., 1992).
The LOAEL identified for
magnesium-induced diarrhea in adults is 360 mg (15 mmol)/day of
magnesium from nonfood sources based on the results of Bashir et
al. (1993). Studies by Fine et al. (1991 b), Marken et al.
(1989), and Ricci et al. (1991) provide evidence to support the
use of this dose as the LOAEL.
Uncertainty Assessment.
Due to the very mild, reversible nature of osmotic diarrhea
caused by ingestion of magnesium salts, an uncertainty factor
(UF) of approximately 1.0 was selected. Unlike possible adverse
effects of other nutrients, osmotic diarrhea is quite apparent to
the individual and thus is not a symptom that is masked until
serious consequences result.
Derivation of the UL.
Because excessive magnesium intake from nonfood sources causes
adverse effects, the UL will be established for magnesium from
nonfood sources. The UL for magnesium for adolescents and adults
is established at 350 mg (14.6 mmol)/day, based on a LOAEL of 360
mg (15 mmol)/day and a UF very close to 1.0. Although a few
studies have noted mild diarrhea and other mild gastrointestinal
complaints in a small percentage of patients at levels of 360 to
380 mg (15.0 to 15.8 mmol)/day, it is noteworthy that many other
individuals have not encountered such effects even when receiving
substantially more than this UL of supplementary magnesium, as
indicated previously.
| UL for
Adolescents and Adults |
> 9
years |
350 mg
(14.6 mmol) of nonfood magnesium |
Infants: Ages 0 to 12 Months
No specific toxicity data exist
on' which to establish a UL for infants, toddlers, and children.
The lack of any available data regarding the effects of magnesium
supplements in infants makes it impossible to establish a
specific UL for infants. Thus, it is important to get magnesium
via food sources only in this age group.
| UL for
Infants |
0 to
12 months |
Not
possible to establish for nonfood magnesium |
Children: Ages 1 through 8 Years
It is assumed that children are as susceptible to the osmotic
effects of nonfood sources of magnesium as are adults. Thus,
adjusting the value for adults on a body-weight basis established
a UL for children at a magnesium intake of 5 mg/kg/day (0.2
mmol/kg/day) (see Table 1-3 for reference weights).
(Page 6-44)
| UL for
Children |
1
through 3 years |
65 mg
(2.7 mmol) of nonfood magnesium |
| |
4
through 8 years |
110 mg
(4.6 mmol of nonfood magnesium |
Pregnancy and Lactation
No evidence suggests increased susceptibility to adverse
effects of supplemental magnesium during pregnancy and lactation.
Therefore, the UL for pregnant and lactating women is set at 350
mg (14.6 mmol)/day-the same value as used for other adults.
| UL for
Pregnancy |
14
through 50 years |
350 mg
(14.6 mmol) of nonfood magnesium |
| UL for
Lactation |
14
through 50 years |
350 mg
(14.6 mmol) of nonfood magnesium |
Special
Considerations
Individuals with impaired renal
function are at greater risk of magnesium toxicity. However, as
noted above, magnesium levels obtained from food are insufficient
to cause adverse reactions even in these individuals. Patients
with certain clinical conditions (for example, neonatal tetany,
hyperuricemia, hyperlipidemia, lithium toxicity, hyperthyroidism,
pancreatitis, hepatitis, phlebitis, coronary artery disease,
arrhythmia, and digitalis intoxication [Mordes and Wacker, 1978])
may benefit from the prescribed use of magnesium in quantities
exceeding the UL in the clinical setting.
Exposure Assessment
In 1986, the most recent year
that data were available to estimate nonfood nutrient supplement
intakes, approximately 15 percent of adults in the United States
reported taking a supplement containing magnesium (although it is
unclear whether supplements were taken on a daily basis) (Moss et
al., 1989). Of those, the ninetieth percentile of daily
supplemental magnesium intake was 200 mg (9.1 mmol) for men and
240 mg (10 mmol) for women; the ninety-fifth percentile was 350
mg (14.4 mmol) for men and 400 mg (16.6 mmol) for women. Thus,
approximately 5 percent of the men and over 5 percent of the
women who used magnesium supplements exceeded the UL of 350 mg
(14.6 mmol)/day in 1986.
Children's intakes from nonfood
nutrient supplements were estimated to be much lower. The
ninetieth percentile of intake for children 2 to 6 years of age
who used magnesium supplements in 1986 was 70 mg (2.9 mmol)/day,
which is approximately the UL for a 2-year- old child weighing 14
kg; the ninety-fifth percentile of intake from supplements was
117 mg (4.9 mmol)/day, or approximately the UL (115 mg [4.8
mmol]/day) for a 6-year-old child weighing 23 kg. Assuming older
children were taking the higher doses, it appears that about 5
percent of the users were exceeding the UL in this study.
(Page 6-45)
Risk Characterization
Using data from 1986, almost 1
percent of all adults in the United States took a nonfood
magnesium supplement that exceeded the reference UL of 350
mg/(14.6 mmol)/day in the 2- week period preceding the survey
(Moss et al., 1989). It is important to note that many of the
individuals whose intakes of supplemental magnesium exceeded the
UL may be self-selectedsiderations
Individuals with impaired renal
function are at greater risk of magnesium toxicity. However, as
noted above, magnesium levels obtained from food are insufficient
to cause adverse reactions even in these individuals. Patients
with certain clinical conditions (for example, neonatal tetany,
hyperuricemia, hyperlipidemia, lithium toxicity, hyperthyroidism,
pancreatitis, hepatitis, phlebitis, coronary artery disease,
arrhythmia, and digitalis intoxication [Mordes and Wacker, 1978])
may benefit from the prescribed use of magnesium in quantities
exceeding the UL in the clinical setting.
Exposure Assessment
In 1986, the most recent year
that data were available to estimate nonfood nutrient supplement
intakes, approximately 15 percent of adults in the United States
reported taking a supplement containing magnesium (although it is
unclear whether supplements were taken on a daily basis) (Moss et
al., 1989). Of those, the ninetieth percentile of daily
supplemental magnesium intake was 200 mg (9.1 mmol) for men and
240 mg (10 mmol) for women; the ninety-fifth percentile was 350
mg (14.4 mmol) for men and 400 mg (16.6 mmol) for women. Thus,
approximately 5 percent of the men and over 5 percent of the
women who used magnesium supplements exceeded the UL of 350 mg
(14.6 mmol)/day in 1986.
Children's intakes from nonfood
nutrient supplements were estimated to be much lower. The
ninetieth percentile of intake for children 2 to 6 years of age
who used magnesium supplements in 1986 was 70 mg (2.9 mmol)/day,
which is approximately the UL for a 2-year- old child weighing 14
kg; the ninety-fifth percentile of intake from supplements was
117 mg (4.9 mmol)/day, or approximately the UL (115 mg [4.8
mmol]/day) for a 6-year-old child weighing 23 kg. Assuming older
children were taking the higher doses, it appears that about 5
percent of the users were exceeding the UL in this study.
(Page 6-45)
Risk Characterization
Using data from 1986, almost 1
percent of all adults in the United States took a nonfood
magnesium supplement that exceeded the reference UL of 350
mg/(14.6 mmol)/day in the 2- week period preceding the survey
(Moss et al., 1989). It is important to note that many of the
individuals whose intakes of supplemental magnesium exceeded the
UL may be self-selected as not experiencing diarrhea, but this is
uncertain. More recent data on estimates of supplement intakes of
a national sample have not been published, but it is unlikely
that usage has declined.
The data on supplement use in
1986 also indicate that at least 5 percent of young children who
used magnesium supplements exceeded the UL for magnesium, 5 mg
(0.2 mmol)/kg/day. However, because less than 10 percent of the
children had taken a magnesium supplement in the past 2 weeks,
less than 1 percent of all children would be at risk of adverse
effects. These estimates assume that older children (with a
higher UL) are taking the higher doses; the percentage at risk
would be higher if dosage were not related to age (and,
therefore, to body size). More information on supplement use by
specific ages is needed.
RESEARCH RECOMMENDATIONS
The ability to determine
reference dietary intakes for magnesium is, as indicated
throughout this chapter, hampered by available data. Areas of
investigation that are particularly needed include the
following:
- Reliable data on population intakes of magnesium are
required based on dietary surveys that include estimates of
intakes from food, water, and supplements in healthy
populations in all life-stages.
- Biochemical indicators that provide an accurate and
specific marker(s) of magnesium status must be investigated in
order to assess their ability to predict functional outcomes
that indicate adequate magnesium status over prolonged
periods.
- Basic studies need to be initiated in healthy individuals,
including experimental magnesium depletion studies that measure
changes in various body magnesium pools in evaluating changes
in possible indicators mentioned above.
- Magnesium balance studies may be one indicator utilized. If
so, strict adherence to criteria suggested in the chapter would
improve their application to dietary recommendations. Moreover,
a determination of the most valid units to use in expressing
estimates of requirements (body weight, fat-free mass, or total
body unit) is needed.
- Investigations are needed to assess the inter-relationships
between dietary magnesium intakes, indicators of magnesium
status, and possible health outcomes that may be affected by
inadequate magnesium intakes, such as hypertension,
hyperlipidemia, atherosclerotic vascular disease, altered bone
turnover, and osteoporosis.
- Based on the evidence of abnormal magnesium status and
health outcomes (as noted above), intervention studies to
improve magnesium status and to assess its impact on specific
health outcomes would be appropriate.
- The toxicity of pharmacological doses of magnesium requires
investigation.
This page was first uploaded to The Magnesium Web Site on
December 12, 1998
http://www.mgwater.com/





