Treatment of Fibromyalgia Syndrome with Super Malic®: A
Randomized, Double Blind, Placebo Controlled, Crossover Pilot
Study
I. JON RUSSELL, JOEL E. MICHALEK, JORGE D. FLECHAS, and GUY
E. ABRAHAM
ABSTRACT. Objective. To
study the efficacy and safety of Super Malic®, a proprietary
tablet containing malic acid (200 mg) and magnesium (50 mg), in
treatment of primary fibromyalgia syndrome (FM).
Methods. Twenty-four
sequential patients with primary FM were randomized to a fixed
dose (3 tablets bid), placebo controlled, 4-week/course, pilot
trial followed by a 6-month, open label, dose escalation (up to 6
tablets bid) trial. A 2-week, medication free, washout period was
required before receiving treatment, between blinded courses, and
again before starting open label treatment. The 3 primary outcome
variables were measures of pain and tenderness but functional and
psychological measures were also assessed.
Results. No clear
treatment effect attributable to Super Malic® was seen in the
blinded, fixed low dose trial. With dose escalation and a longer
duration of treatment in the open label trial, significant
reductions in the severity of all 3 primary pain/tenderness
measures were obtained without limiting risks.
Conclusions. These data
suggest that Super Malic® is safe and may be beneficial in
the treatment of patients with FM. Future placebo-controlled
studies should utilize up to 6 tablets of Super Malic bid and
continue therapy for at least 2 months. (J Rheumatol
1995;22:953-8)
Key Indexing Terms:
FIBROMYALGIA MAGNESIUM MALIC ACID TREATMENT ATP
The development of new therapies for fibromyalgia (FM) has
been substantially impaired by the lack of a proven etiology or
pathogenesis for the disorder1. Clinicians typically
employ various combinations of medications, exercise, rest, and
psychological support. Limited benefit has been observed in
controlled studies with 3 psychotropic drugs:
amitriptyline2-4, cyclobenzaprine5,6, or
alprazolam7 in relatively low dosage. Propionic acid
nonsteroidal antinflammatory drugs, such as ibuprofen or naproxen
have shown promise in combination with sedative hypnotic drugs
but not as sole therapy8. While such chemical agents
do exhibit some analgesic, anxiolytic, sedative, and
antidepressant effects, the mechanisms responsible for their
influence on FM symptoms are still uncertain.
One proposed explanation for the soft tissue pain experienced
by patients with FM was that muscle energy metabolism may be
compromised9-16. That possibility is not
excluded by normal skeletal muscle
histologically17. It has even been suggested that
abnormal blood flow may deprive muscle of sufficient
oxygen18 and other nutrients. Histochemical analysis
indicates that tender points in FM muscle are deficient in high
energy phosphate (ATP)19. Nuclear magnetic resonance
spectroscopy has suggested abnormal high energy phosphate
metabolism in exercising FM muscle but that may be an artifact of
poor physical conditioning21. More recently, our group
has shown that red blood cells from FM are deficient in ATP
relative to matched normal controls22. Muscle
magnesium may also be deficient in FM23.
Malic acid, a naturally occurring, nontoxic, organic
dicarboxylic acid, and magnesium are both known to be involved in
the processes of generating ATP24. They play a pivotal
role in mitochondrial ATP synthesis24. A rationale for
the combined use of malic acid and magnesium in the treatment of
FM was proposed by Abraham and Flechas24. They also
reported dramatic relief from pain in FM with short term, open
label administration of Super Malic®, which contains malic
acid and magnesium hydroxide. The present study was designed to
more rigorously test their hypothesis.
MATERIALS AND METHODS
Patients. Twenty-nine sequential patients with FM
were evaluated at the screening visit (week -2) and found to meet
the American College of Rheumatology (ACR) criteria25
for primary FM. After informed consent, patients were asked to
discontinue all medications used to treat PM symptoms. The
protocol was approved and monitored by the Institutional Review
Board.
Medications. Super Malic (Optimox Corporation,
Torrance, CA) contains a proprietary formulation of malic acid
(200 mg) and magnesium hydroxide (50 mg)/tablet and is available
without prescription in CA. The manufacturer provided an
identically coated placebo that matched Super Malic with regard
to appearance and taste. Acetaminophen was allowed under self-
control at a dosage of 325 mg to 650 mg once/day for severe
headache. No other analgesic or sedative hypnotic drugs were
allowed during the initial placebo controlled trial. Patients
were allowed to reintroduce prior therapies while continuing
Super Malic during the final 4 months of the open label trial as
indicated below.
Interventions. We describe 2 related trials: (1)a
randomized, double blind, placebo controlled, 2-treatment period,
crossover trial, and (2) a subsequent 6-month, open label,
followup trial, in which the effects of Super Malic dosage
escalation were assessed before and after resumption of prestudy
medication.
Coded label trial. At entry into the initial placebo
controlled trial, patients with FM were required to have been
free of analgesic and sedative-hypnotic medications for the 2
weeks prior to baseline measurements. Those who successfully
discontinued prestudy medications were randomized to 1 of the 2
treatment sequences: 12 patients to Super Malic followed by
placebo (sequence MP), and 12 to placebo followed by Super Malic
(sequence PM). They took 3 coded tablets bid for 4 weeks,
followed by a 2-week washout and crossover to a 4-week treatment
period with the other coded tablet at the same dosage. All
clinical assessments were obtained by a single examiner UR) at
the beginning and end of each 4-week trial period.
Open label trial. After completion of the placebo
controlled trial, patients underwent another 2-week, medication
free, washout period before beginning the open label trial. They
then started therapy with 3 tablets of Super Malic bid and
increased the dosage every 3-5 days until they experienced
acceptable benefit or developed a symptom suspected of being a
treatment related side effect.
After Month 2 of this open label trial, patients were allowed
to reintroduce medications used for FM treatment before study
entry. The purposes were to determine (1) whether patients would
elect to resume their prior medications, and (2) whether
continued treatment with Super Malic might increase the apparent
effectiveness of those medications.
Outcome assessment. All 6 outcome measures used had
been validated in prior studies. The 3 primary outcome measures
were (I) patient self- assessment of pain on a 10 cm visual
analog scale (VAS) (pain), (2) the tender point index (TPI),
which is the sum of individual tenderness severities26
at each of the 18 standard ACR tender points, and (3) the tender
point average (TPA), which is the mean value derived from
dolorimeter readings (in kg) at each of the 18 ACR tender
points
Three variables of secondary importance included (1) the
Health Assessment Questionnaire (HAQ) score, (2) the Center for
Epidemiologic Studies-Depression (CESD) Scale score and (3) the
Hassle Scale score (Hassle)
Adverse effects. Any adverse event experienced was
self-reported on the daily diary and later transferred by study
staff to the case report form.
Sample size. Prospective sample size determinations
were based on the results of a prior study conducted by the same
team7. In that study, 15 patients receiving both
ibuprofen and alprazolam experienced an average 7-point (30%)
drop in TPI by the end of the blinded treatment period (Week 6).
A parallel group of 14 patients receiving double placebo
experienced an average 4.4-point (17.5%) drop in TPI. The
standard deviations of the Week 0 to Week 6 changes in these 2
groups were 7.7 and 6.4, respectively.
Based on the results of a pilot study24, it was
assumed Super Malic would be more effective than combined
alprazolam and ibuprofen7. Pretreatment calculations
indicated that 20 patients who completed both Super Malic and
placebo interventions would provide 89% power for detecting a
significant (p ≤ 0.05) 8-point (34%) difference in
TPI between active and placebo interventions. The null hypothesis
predicted that there would be no difference. To accommodate an
estimated 20% loss to followup, the design called for a minimum
of 24 patients at baseline.
Statistical analysis. SAS (SAS Institute, Cary, NC,
1993) software was used for analysis of variance and
x2 tests. p Values > 0.05 and <
0.10 were considered borderline significant. Ninety-five percent
confidence intervals for treatment group mean differences on
continuously distributed variables were computed from least
square means and the associated mean square error. All
statistical tests were 2-sided at the 5% significance level.
The primary assessment of outcome for the study was based on
the results from the blinded, placebo controlled trial, while the
open label trial used the same measures to explore effects of
Super Malic dosage titration and of its coadministration with
more traditional medicinal therapies.
Analyses of placebo controlled trial followed the repeated
measures analysis of variance procedure described by Ratkowsky,
Evans, and Alldredge31. These analyses incorporated
outcomes measured at each of 5 periods: at the end of the
prestudy washout period (baseline, Week 0) at the end of the
first treatment period (Week 4), at the end of the 2nd washout
(Week 6), at the end of the 2nd treatment (Week 10), and at the
end of the 3rd washout (Week 12). The output was in “mean
response” and “mean carry over
effect”/treatment period. This approach has the advantage
of increased efficiency over analyses using fewer measurements,
providing uncorrelated estimates of treatment and carryover
effects. All analyses assume first order carryover effects,
meaning that subsequent manifestations of treatment, if they
existed, would not affect measurements taken more than 1 period
later.
The assessment of efficacy during the open label trial
individually compared responses at Months 2, 4 and 6 with the
baseline values obtained following the final washout at Week 12
(redefined for the open label trial as Month 0 baseline).
Possible adverse effects from the study intervention and the
influence of concomitant medications resumption during the open
label trial were evaluated descriptively.
RESULTS
Of the 29 patients with FM who met study criteria at the
screening visit (Week -2), 5 were excluded before the Week 0
baseline visit because they were unable to discontinue prestudy
medications. Of the 24 patients with FM who were randomized, 4
were withdrawn before Week 10. Three of these decided not to
continue at varying times between Week 0 and Week 4. The 4th
patient dropped out after Week 6 but before Week 10.
Table 1 summarizes the baseline values for each of the
demographic and outcome variables assessed among the 24 patients
with FM who entered the coded trial and the 18 patients with FM
who entered the open label trial. In separate analysis,
demographic and clinical values from the 16 patients who
completed both trials were compared with measures from the 13
subjects who dropped out at some point after the baseline (Week
0) evaluation. The dropouts did not significantly differ from the
completing study patients with respect to any of the baseline
variables (data not shown).
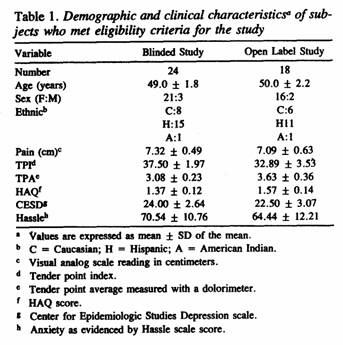
The patients in this study were more severely affected than
those in an earlier study performed in the same
community7. The average values for pain (present study
mean ± SD vs prior study: 7.3 ± 0.5 vs 6.1 ±
0.2), TPI (37.5 ± 2.0 vs 25.7 ± 1.1), and TPA (3.l
± 0.2 vs 4.1 ±0.1) were numerically worse
among patients in the present trial. That was also true for the
secondary variables. While the HAQ (1.4 ± 0.1 vs 1.3
± 0.1) was only slightly higher, the CESD score (24.0
± 2.6 vs 9.4 ± 0.6) was substantially higher. The
distribution of scores on the CESD indicated that 62% of the 24
patients with FM had values greater than or equal to 18, which
can be interpreted as indicating “possible clinical
depression,” compared with only 5% in the previous
trial7.
Baseline analyses on the blinded trial’s randomization
into the 2 crossover treatment sequence groups showed that the 2
cohorts were comparable with regard to age, sex, ethnicity, and
all of the baseline values from the clinical outcome
measures.
No protocol violations were judged to have occurred for any
randomized patient throughout the 2 trials. A few patients took
allowed rescue medication (acetaminophen 325 mg, 1-2 doses in 24
hours) for headache or severe muscular pain, but most did
not.
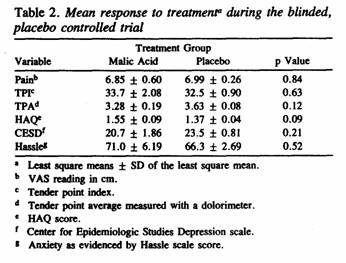
The treatment responses for each of the 3 primary and 3
secondary outcome measures in the blinded treatment trial are
shown in Table 2. There were no significant carryover effects
from placebo to Super Malic or vice versa. The data consistently
failed to show significantly greater improvement during the Super
Malic treatment period than during the placebo treatment period
(see also Figure 1). Factoring in the usage of acetaminophen did
not alter the results.
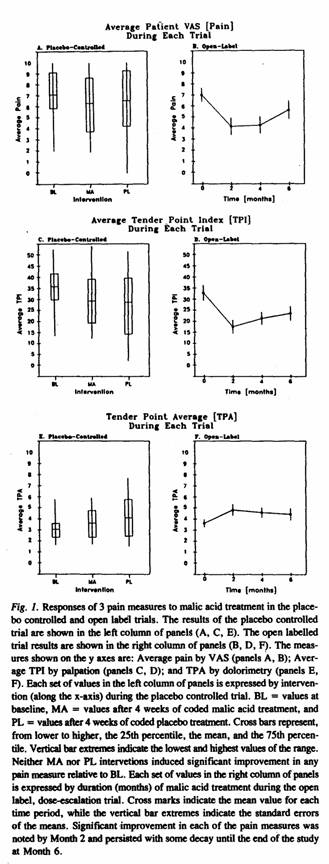
Eighteen of the 20 patients with FM who completed the coded
trial entered the open label trial. One of those dropped out
after Month 2; and another failed to take sufficient Super Malic
on a vacation trip after Month 2. Thus, data from 18 patients
were subjected to analysis at Month 2 and thereafter from 16
patients.
From Month 0 to Month 2 of the open label trial, the dosage of
the Super Malic was raised until mean daily dosage at Month 2 was
7.2 tablets/day (range 3-10/day, with 4 taking 10/day), at Month
4 the mean dosage was 8.6 tablets/day (range 4-12/day, with 7
taking 10/day and 1 taking 12/day and at Month 6, the mean dosage
was 8.8 tablets/day (range 4-14/day, with 6 taking 10/day, 1
taking 11/day, and 1 taking 14/day).
Some patients resumed prestudy medications after Month 2 as
allowed in the open label trial protocol. At the Month 4 and 6
visits, 8 of the remaining 16 patients were taking such
medication (e.g., by Month 4, 1 had resumed cyclobenzaprine 10 mg
hs, 1 alprazolam 0.5 mg hs, 1 cortisone acetate 25 mg daily, 2
methocarbomal 780 mg hs, and 3 took ibuprofen 800-2,400
mg/day).
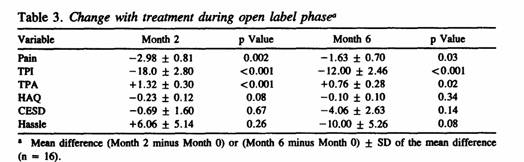
The mean values for the main outcome variables used in the
open label trial are shown in Table 3. They reflect change with
treatment for the 3 primary and 3 secondary outcome measures at
Months 2 (n = 18) and 6 (n = 16). Significant improvements were
observed at Month 2 in all 3 primary variables (pain, TPI, TPA)
but not in any of the 3 secondary variables. Corresponding
analysis of the same measures at Month 6 indicated persistence of
improvement in all of the primary variables (see also Figure
1).
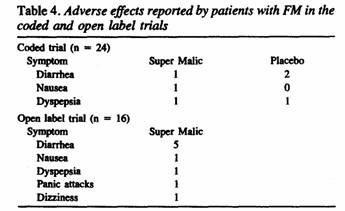
A descriptive summary of adverse effects reported by patients
is shown in Table 4. It indicates very low risk associated with
the malic acid treatment. In fact, it was not clear that any
adverse event was really treatment related. Of the 24 randomized
patients, 13 recorded at least 1 adverse event during the 2
trials. When the code was broken on the blinded trial, it became
apparent that none of the adverse events could be attributed to
malic acid and/or magnesium. Gastrointestinal symptoms seemed to
predominate, especially during the open label trial. Looser
stools provided welcome relief from constipation in 2 cases while
in 3 other patient they were easily managed by addition of bulk
containing agents.
DISCUSSION
Malic acid is a naturally occurring asymmetric, organic,
dicaboxylic acid molecule that exists in dextrorotatory (D) and
levorotary (L) forms. It is widely distributed in the vegetable
kingdom including concentrations of 4 to 8 g/l of apple juice,
90% being in L-configuration and 10 in the D-form Malic acid is
available commercially in a racemic mixture with an LD of 3600
mg/kg body weight34.
Combinations of magnesium-hydroxide and malate have a wide
margin of safety. Magnesium hydroxide compared favorably to the
citrate, lactate and chloride form of magnesium in terms of
bioavailability when administered orally35. Magnesium
hydroxide at an oral dosage of 500 mg/day significantly increased
muscle magnesium levels36. Super Malic was formulated
to favor lymphatic absorption, mimicking parenteral
administration. For a 50 kg person ingestion of 6 tablets of
Super Malic would correspond to 24 mg of malate/kg body
weight.
Using swimming time to exhaustion as an index of physical
endurance, the optimal response in rats was observed with an oral
dosage of 250 mg/kg body weight37. However
mitochondrial respiration was stimulated with only 7.5 mg/kg body
weight when intraperitoneal administration was
used38.
All of the patients in this study met the published ACR
criteria for primary FM25, 26 and exhibited the
typical clinical features of that disorder. The patients were
otherwise typical of FM populations with a female:male ratio of
7:1, and mean age of 49 years. The ethnic dist (62.5% Hispanic)
was comparable to that of the overall San Antonio community
(about 60% Hispanic). The greater severity of symptoms in this
trial relative to the earlier one at this site may have resulted
from a large number of tertiary referrals to IJR.
Sample size calculations for the double blind, placebo
controlled, crossover study predicted that 20 patients with FM
would be adequate to demonstrate a significant improvement with
malic acid relative to placebo. This estimate was about one-third
more conservative than the figure predicted by Abraham and
Flechas24 with patients with FM given 600 mg malic
acid twice daily. Therefore, the sample size chosen was
considered realistic. Furthermore, the dropout rate in our study
was exactly as predicted.
While the blinded, placebo controlled trial failed to show
significant improvement in the 3 main outcome measures, the
results from the open label trial were very encouraging. We
attribute this pattern to use of a higher dosage and longer
duration of treatment with Super Malic during the open label
trial. Although about half of the patients in the open label
trial resumed prestudy medications after Month 4, no clear
benefit could be attributed to them since the overall change in
symptoms from the Month 0 baseline was no greater at Month 6 than
at Month 2.
None of the 3 secondary outcome measures displayed any
response to coded Super Malic therapy. The borderline significant
treatment effect in the HAQ appeared to result from a
surprisingly strong placebo effect in the cohort first randomized
to placebo and, therefore, cannot be counted as an indicator of
malic acid efficacy.
The results of open label treatment with Super Malic seem to
indicate that it may be beneficial on the painful FM symptoms in
dosages in excess of 8 tablets/day for up to 6 months. Some
tachyphylaxis cannot be excluded because concomitant medications
were allowed during the last 4 months. A similar result was
obtained with open label alprazolam and ibuprofen Therefore, one
must wonder whether the observed improvement in both open label
trials resulted in part from hopeful optimism. For whatever
reason, benefit occurred while patients were receiving open label
malic acid and magnesium. It is appropriate to recall that most
medical care is also open label and this adjuvant therapy was
associated with a minimum of risk.
Since the blinded trial results were not statistically
significant, we cannot reject the null hypothesis for the 3
tablets bid dosage of Super Malic and we cannot confidently
attribute the open label findings solely to the malic
acid/magnesium combination. However, the benefits observed during
the open label trial suggest that Super Malic may prove
efficacious in a longer study in which it is administered in a
higher daily dosage.
The mechanism by which malic acid and magnesium might be
beneficial in patients with FM remains uncertain. Several
possible roles for this combination were proposed by Abraham and
Flechas24 to involve alterations in carbohydrate
metabolism that would lead to increased production of ATP.
Indeed, a series of recent reports detail the discovery of
defects in carbohydrate metabolism among patients with FM42,
43, There is also evidence to suggest that energy
generation may not be normal in FM since ATP is low in muscle
tender points19 and in red cells22.
ACKNOWLEDGMENT
The authors thank Dani Presswood for editorial assistance.
REFERENCES
1. Russell IJ: Fibrositis/fibromyalgia syndrome. In: Hyde
BMII, Goldstein JA, Levine PH, eds. The Clinical and
Scientific Basis of Myalgic Encephalomyelitis Chronic Fatigue
Syndrome. Ottawa: The Nightingale Research Foundation,
1992:663-90.
2. Goldenberg DL, Felson DT, Dinerman H: A randomized,
controlled trial of amitriptyline and naproxen in the treatment
of patients with fibromyalgia. Arthritis Rheum 1986;29:
1371-7.
3. Scudds RA, McCain GA, Rollman GB, Harth M: Improvements in
pain responsiveness in patients with fibrositis after successful
treatment with amitriptyline. J Rheumatol
1989;19:98-103.
4. Jaeschke R, Adachi J, Guyatt G, Keller J, Wong B: Clinical
usefulness of amitriptyline in fibromyalgia: the results of 23
N-of-1 randomized controlled trials. J Rheumatol
1991:18:447-51.
5. Bennett RM, Gatter RA, Campbell SM, Andrews RP, Clark SR,
Scarola JA: A comparison of cyclobenzaprine and placebo in the
management of fibrositis. A double-blind controlled study.
Arthritis Rheum 1988:31:1535-42.
6. Reynolds WJ, Moldofsky H, Saskin P. Lue PA: The effects of
cyclobenzaprine on sleep physiology and symptoms in patients with
fibromyalgia. J Rheumatol 1991:18:452-4.
7. Russell IJ, Fletcher EM, Michalek JE, McBroom PC, Hester
GO: Efficacy and safety of ibuprofen and alprazolam in the
treatment of primary fibromyalgia/fibrositis syndrome: A
double-blind placebo-controlled study. Arthritis Rheum
1991;34:552-60.
8. Yunus MB, Masi AT, Aldag JC: Short term effects of
ibuprofen in primary fibromyalgia syndrome: a double blind,
placebo controlled trial. J Rheumatol
1989;166:527-32.
9. Bennett RM, Clark SR, Goldberg L, et al: Aerobic fitness in
patients with fibrositis. A controlled study
of respiratory gas exchange and l33xenon clearance from
exercising muscle. Arthritis Rheum
1989;32:454-60.
10. Mengshoel AM, Forre O, Komnaes HB: Muscle strength and
aerobic capacity in primary fibromyalgia. Clin Exp
Rheumatol 1990;8:475-9.
11. Henriksson KG: Muscle pain in neuromuscular disorders and
primary fibromyalgia. Eur J Appl Physiol
1988;57:348-52.
12. Elert JE, Rantapaa-Dahlqvist SB, Henriksson-Larsen K,
Gerdle B: Increased EMO activity during short pauses in patients
with primary fibromyalgia. Scand J Rheumatol
1989;l8:321-3.
13. Elert JE, Rantapaa-Dahlqvist SB, Henriksson-Larsen K,
Lorentzon R, Gentle BU: Muscle performance, electromyography and
fibre type composition in fibromyalgia and work-related myalgia.
Scand J Rheumatol 1992;21 :28-34.
14. Elert JE, Rantapaa-Dahlqvist SB, Henriksson-Larsen K,
Gerdle B: Increased EMG activity during short pauses in patients
with primary fibromyalgia. Scand J Rheumatol
1989;l8:321-3.
15. Bengtsson A, Cederblad 0, Larsson J: Carnitine levels in
painful muscles of patients with fibromyalgia (letter). Clin
Exp Rheumatol 1990;8:197-8.
16. Zidar J, Backman E, Bengtsson A, Henriksson KG:
Quantitative EMG and muscle tension in painful muscles in
fibromyalgia. Pain 1990;40:249-54.
17. Yunus MB, Kalyan-Raman UP, Masi AT, Aldag JC: Electron
microscopic studies of muscle biopsy in primary fibromyalgia
syndrome: A controlled and blinded study. J Rheumatol
1989;l6:97-101
18. Lund N, Bengtsson A, Thorborg P: Muscle tissue oxygen
pressure in primary fibromyalgia. Scand J Rheumatol
1986
19. Bengtsson A, Henriksson KG, Larsson J: Reduced high energy
phosphate levels in the painful muscles of patients with primary
fibromyalgia. Arthritis Rheum 1986;29:817-21.
20. de Blecourt AC, Wolf RF, van Rijswijk MH, Kamman RL,
Knipping AA, Mooyaart EL: In vivo 31P magnetic resonance
spectroscopy (MRS) of tender points in patients with primary
fibromyalgia syndrome. Rheumatol Int 1991;11:51-4.
21. Simms RW, Roy S, Skrinar G, et al: (31)P-NMR spectroscopy
of muscle in fibromyalgia syndrome patients and sedentary
controls. Arthritis Rheum 1993;37:794-800.
22. Russell IJ, Vipraio GA, Abraham GE: Red cell nucleotide
abnormalities in fibromyalgia syndrome (abstr). Arthritis
Rheum 1993;36:S223.
23. Clauw D, Ward K, Katz P, Rajan S: Muscle intracellular
magnesium levels correlate with pain tolerance in fibromyalgia
(FM) (abstr). Arthritis Rheum 1994;37:R29.
24. Abraham GE, Flechas JD: Management of fibromyalgia:
Rationale for the use of magnesium and malic acid. J Nutrit
Med 1992;3:49-59.
25. Wolfe F, Smythe HA, Yunus MB, et al: The American College
of Rheumatology 1990 Criteria for the Classification of
Fibromyalgia. Arthritis Rheum 1990;33:160-72.
26. Russell IJ, Vipraio GA, Morgan WW, Bowden CL: Is there a
metabolic basis for the fibrositis syndrome? Am J
Med 1986:81:50-6.
27. Dinerman H, Goldenberg DL, Felson DT: A prospective
evaluation of 118 patients with the fibromyalgia syndrome:
prevalence of Raynaud’s phenomenon, sicca symptoms, ANA,
low complement, and Ig deposition at the dermal-epidermal
junction. J Rheumatol 1986;13:368-73.
28. Fries JF, Spitz F, Young DY: The dimensions of health
outcomes: The health assessment questionnaire, disability and
pain scales. J Rheumatol 1982,
29. Radloff LS: The CES-Scale: A self-report depression scale
for research in the general population. Applied Physiological
Measurements. Appl Physiol Meas 1977;1:385-401.
30. Dailey PA, Bishop GD, Russell IJ, Fletcher EM:
Psychological stress and the fibrositis/fibromyalgia syndrome.
J Rheumatol 1990;17:1380-5.
31. Ratkowsky DA, Evans MA, Alldredge JR: Cross-Over
Experiments: Design. Analysis, and Application. New York:
Marcel Dekker, 1993:110-5.
32. Lee HS, Wrolstad RE: Apple juice composition: Sugar,
nonvolatile acid, and phenolic profiles. J Assoc Anal
Chem 1988;7l:789-94.
33. Patel DM, Popp GT, Williamson S, Lund W: Malic acid. In:
Handbook of Pharmaceutical Excipients. Washington: American
Pharmaceutical Association and The Pharmaceutical Society of
Great Britain, London, England, 1986:176.
34. Domingo JL, Gomez JM, Llobet JM, Corbella J: Comparative
effects of several chelating agents on the toxicity, distribution
and excretion of aluminum. Hum Toxicol
1988;7:259-62.
35. Bohmer T, Roseth A, Holm H, Weberg-Teigen S, Wahl L:
Bioavailability or oral magnesium supplementation in female
students evaluated from elimination of magnesium in 24-hour
urine. Magnesium Trace Elem 1990;9:272-8.
36. Sjogren A, Floren CH, Nilsson A: Oral administration of
magnesium hydroxide to subjects with insulin-dependent diabetes
mellitus: Effects on magnesium and potassium levels and on
insulin requirements. Magnesium 1988;7: 117-22.
37. Dunaev VV, Tishkin VS, Milonova NP, Belay IM, Makarrenko
AN, Garmash SN: Effect of malic acid salts on physical working
capacity and its restoration after exhausting muscular work.
Farmakol Toksikol 1988;51 :21-5.
38. Bobyleva-Guarriero V, Lardy HA: The role of malate in
exercise-induced enhancement of mitochondrial respiration.
Arch Biochem Biophys 1986:245:470-6. 1
39. Campbell SM, Clark S, Tindall EA, Forehand ME, Bennett RM:
Clinical characteristics of fibrositis. I. A
“blinded,” controlled study of symptoms and tender
points. Arthritis Rheum 1983;26:817-24.
40. Yunus M, Masi AT, Calabro JJ, Miller KA, Feigenbaum SL:
Primary fibromyalgia (fibrositis): Clinical study of 50 patients
with matched normal controls. Semin Arthritis Rheum
1981:11:151-71.
41. Clark S, Campbell SM, Forehand ME, Tindall EA, Bennett R
Clinical characteristics of fibrositis. II. A
“blinded”: controlled study using standard
psychological tests. Arthritis Rheum 1985;28:132-7.
42. Eisinger J, Ayavou T: Transketolase stimulation in
fibromyalgia. J Am Coll Nutr 1990;9:56-7.
43. Eisinger J, Plantamura A, Ayavou T: Glycolysis
abnormalities in fibromyalgia. J Am Coll Nutr
1994;13:144-8.
From the Rheumatology Section, Division of Clinical
Immunology, Department of Medicine, The University of Texas
Health Science Center, San Antonio, Texas, USA.
Supported by a grant from OPTIMOX Corporation, Suite 406,
2720 Monterey Street, Torrance, Ct 90503.
I.J. Russell, MD. PhD, Associate Professor of Medicine,
UTHSC, San Antonio; J.E. Michalek, PhD, Director Airforce Health
Study, Armstrong Laboratory, Brooks Air Force Base, TX; J.D.
Flechas, MD, MPH, private family practice, Hendersonville, NC;
G.E. Abraham, MD, Medical Director, OPTIMOX Corporation,
Torrance, CA, USA.
Address reprint requests to Dr. I.J. Russell, Department
of Medicine, University of Texas Health Science Center, 7703
Floyd Curl Drive, San Antonio, 7X 78284-7874.
Submitted July 8, 1994 revision accepted November 14,
1994.
This page was first uploaded to The Magnesium Web Site on July
20, 200
http://www.mgwater.com/





